Application of 13-hydroxy-8,9-dehydroshizukanolide in preparing medicine for anti-platelet aggregation
An anti-platelet aggregation, caryophyllol technology, applied in the field of biomedicine, to achieve excellent anti-platelet aggregation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 1. Experimental materials
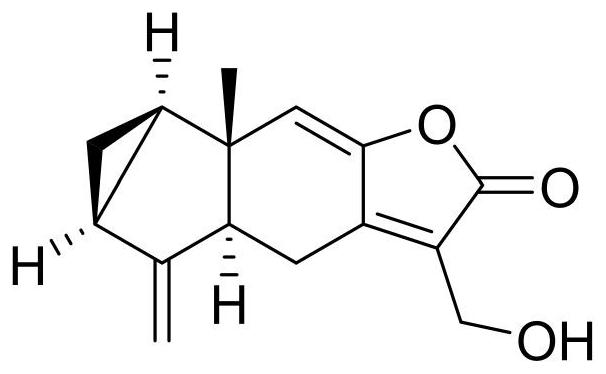
[0018] The chemical structure of 13-hydroxy-8,9-dehydrocaryophyllol is as follows figure 1 As shown, the purity is 98.5%.
[0019] The body weight of New Zealand male rabbits is (2±0.2) kg, and they are ready for use after one week of adaptive feeding.
[0020] 2. Experimental method
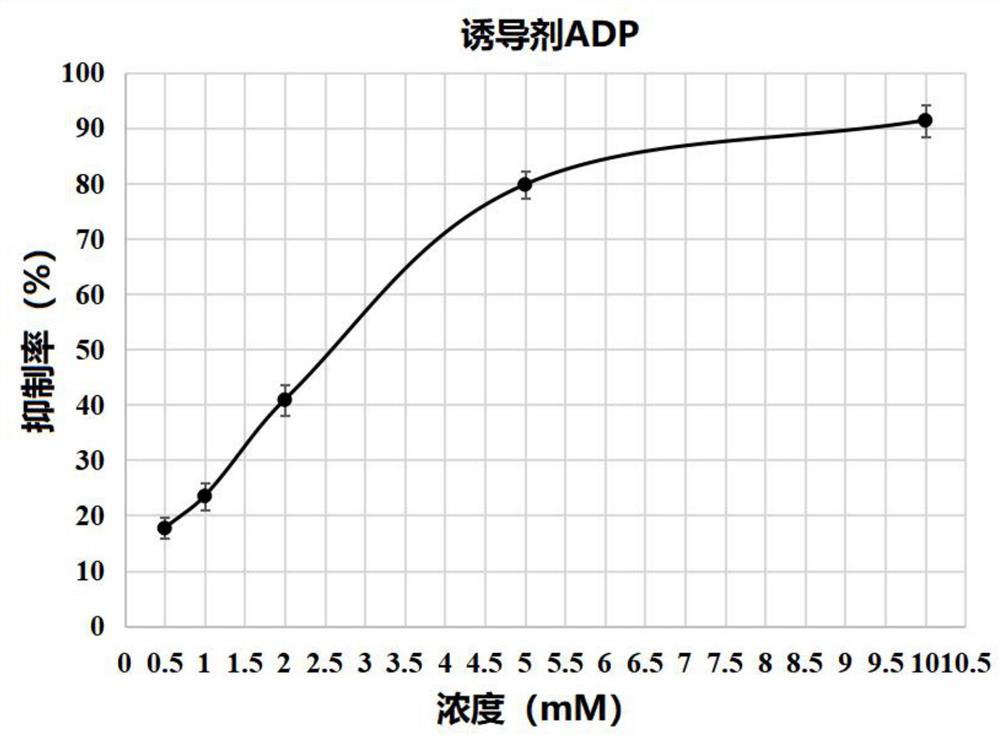
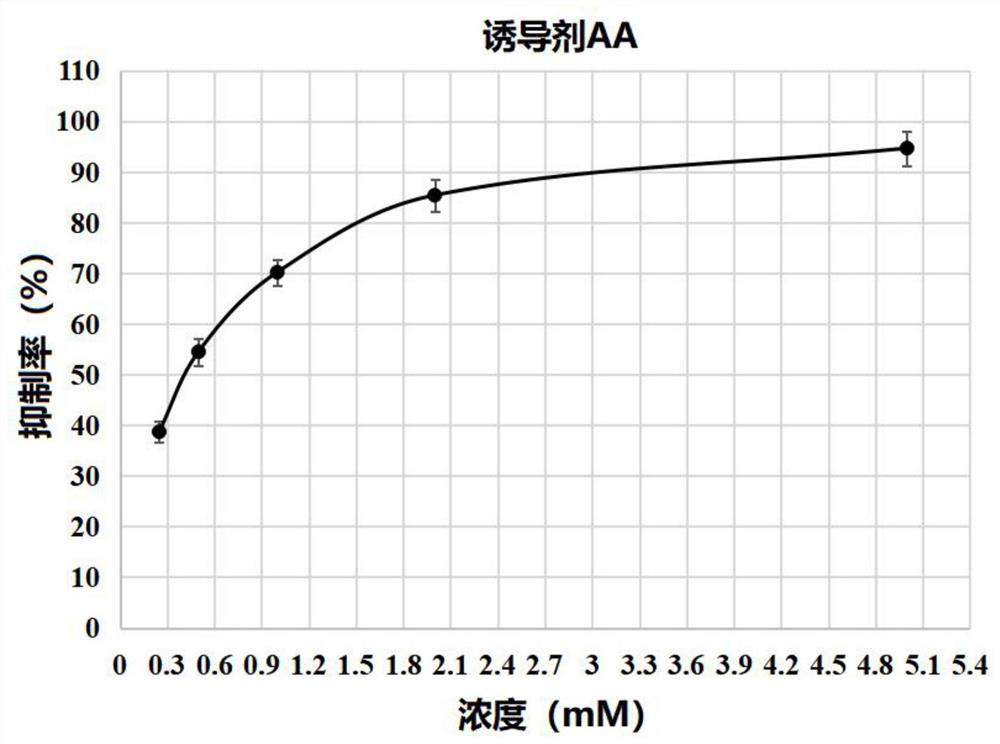
[0021] The in vitro anti-platelet aggregation activity assay method is the Born turbidimetric method, with aspirin as a positive control drug, the specific operation method reference (Tang Quan et al., 13-hydroxy-8,9-dehydrocaryophyllol design, synthesis and anti-platelet aggregation research , 2020):
[0022] Locally anesthetize the rabbit with 1% procaine hydrochloride, intubate the carotid artery to take blood, put it into 3.8% sodium citrate solution (the volume ratio of the blood taken to the sodium citrate solution is 9:1), Centrifuge at 1000r / min for 15min to obtain platelet-rich plasma (PRP). Dissolve 13-hydroxy-8,9-dehydrocaryophyllol and aspirin ...
Embodiment 2
[0030] An anti-platelet aggregation tablet, capsule or injection, the active ingredient is 13-hydroxy-8,9-dehydrocaryophyllol in different contents, and the excipients are commonly used excipients for tablets or capsules or injections.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com