Cinnamamide derivatives as well as preparation method and medical application thereof
A technology of phenylacrylamide and derivatives, applied in the field of phenylacrylamide derivatives or pharmaceutically acceptable salts thereof and the preparation thereof, can solve problems such as disappointing results, achieve high yield, significant anti-platelet aggregation activity, structural novel effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
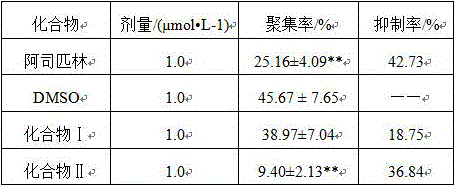
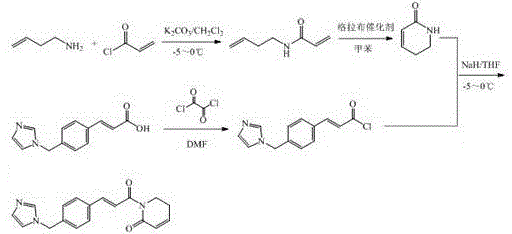
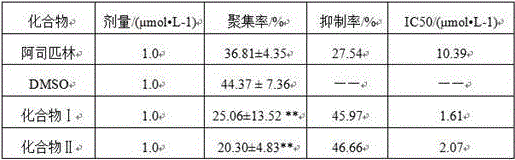
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of compound I
[0029] 1000ml dry three-necked flask, install stirrer, thermometer, add 3-buten-1-amine (28.5g,
[0030] 0.4mol), anhydrous K 2 CO 3 (58g, 0.42mol) and 310ml of dry dichloromethane, cooled to -5°C ~ 0°C, under stirring, slowly add 100ml of acryloyl chloride (36.5g, 0.4mol) in dichloromethane solution, after the addition is complete, keep warm and stir to react 1h, then warmed up to room temperature and stirred for 7h, TLC identification of the reaction end point [thin layer development conditions (petroleum ether-ethyl acetate = 10:1)], the reaction was completed, filtered, the filtrate was washed with water (3 × 50ml), anhydrous Na 2 SO 4 After drying, the desiccant was removed by filtration, and the filtrate <40°C was concentrated to dryness under reduced pressure to obtain 41.6 g of light yellow oily liquid (Intermediate 1), with a yield of 83%, which was directly used in the next step.
[0031] Intermediate 1 (41.6g,...
Embodiment 2
[0034] Embodiment 2: the preparation of compound I tartrate
[0035] Compound Ⅰ (10g, 0.0325mol) and ethanol 50ml, at room temperature, add tartaric acid (5.3g, 0.035mol),
[0036] Heat to 45°C-50°C, stir for 20min, cool to 0°C-5°C, stand overnight, filter, wash the solid with cold ethanol, and dry it under vacuum at 45°C-50°C to obtain 11.6g of white powdery solid, HPLC content 99.4%.
Embodiment 3
[0037] Embodiment 3: the preparation of compound II
[0038] Intermediate 3 (20g, 0.081mol), 120ml of anhydrous tetrahydrofuran, heated to reflux, at this time, added hexamethyldisilazane (14.5g, 0.09mol) and 11ml of triethylamine, stirred and refluxed overnight, concentrated under reduced pressure To dryness, the residue was extracted with dichloromethane (3×200ml), the combined organic layers were washed with water (3×50ml) and saturated brine (2×50ml), anhydrous Na 2 SO 4 After drying, the desiccant was removed by filtration, concentrated to dryness under reduced pressure, and recrystallized from isopropanol to obtain 9.7 g of compound II as a yellow powder solid, with a yield of 53%, mp: 236-241°C, and HPLC content of 98.2%. EIS-MS, m / z: 452[M+H] + ; 1 H NMR (400MHz, CH 3 OH-d 3 / TMS): δ H (ppm): 7.65 (t, J=15.8Hz, 2H), 7.19~7.38(m, 8H), 7.11~7.17(m, 4H), 7.02~7.10(m, 2H), 6.83(d, J=6.5 Hz, 2H), 5.57 (s, 4H), 3.39 (d, J=5.9Hz, 4H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com