Method for synthesizing 1, 5-dinitroanthraquinone by continuous flow micro-channel reactor
A technology of microchannel reactor and channel reactor, which is applied in chemical instruments and methods, chemical/physical/physicochemical reactors, preparation of nitro compounds, etc., can solve unstable reaction process, high production risk and long reaction time and other problems, to achieve the effect of convenient intelligent production, excellent heat transfer performance and rapid response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
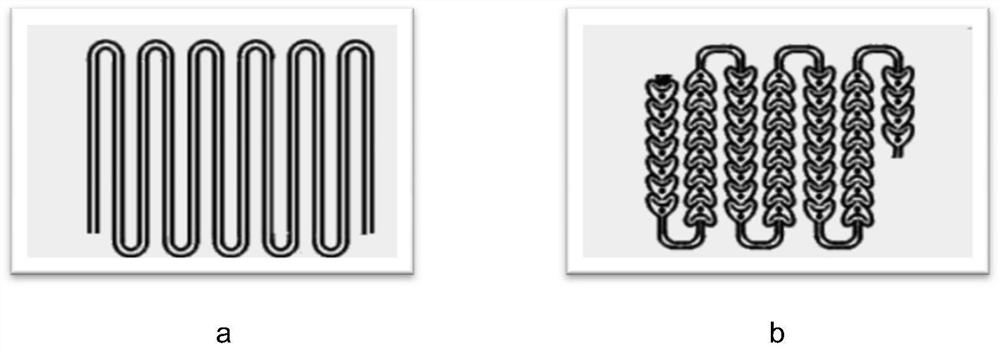
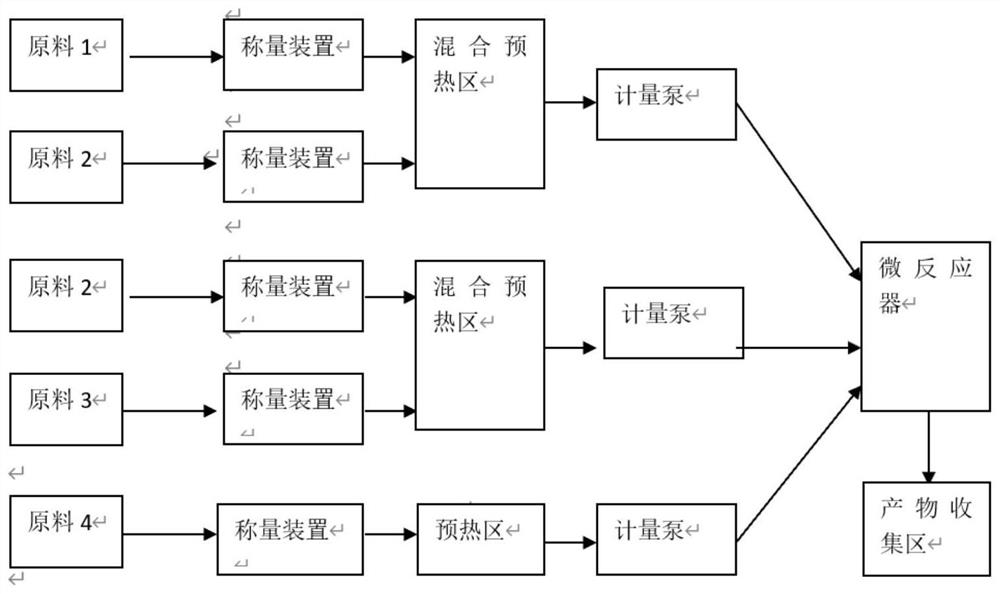
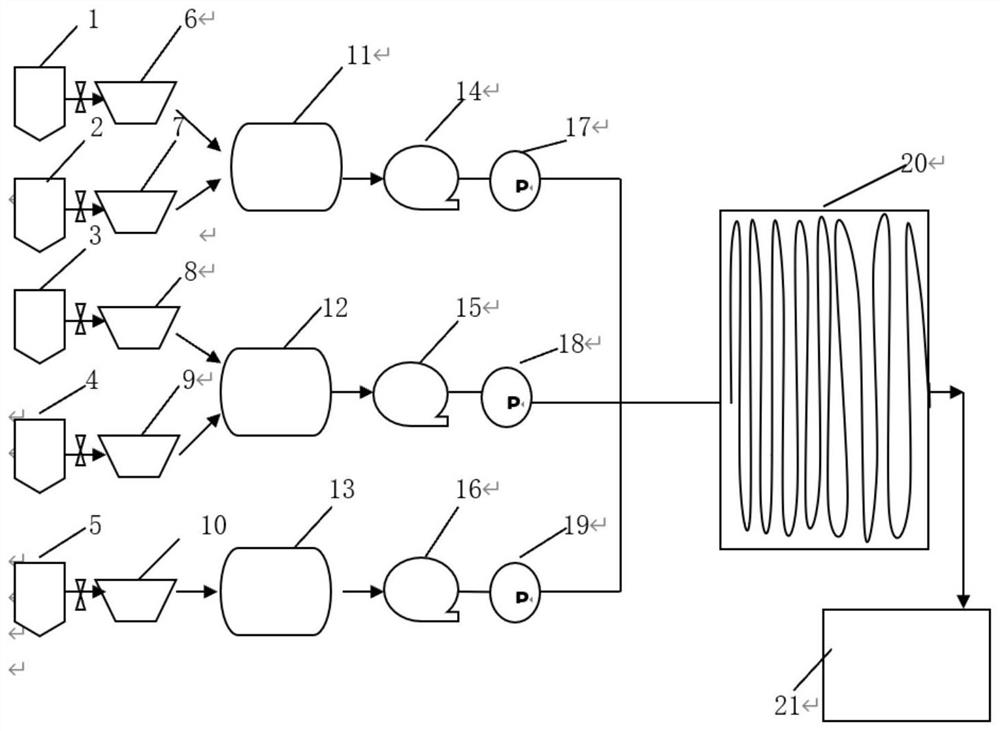
[0026] (1) Refer to figure 2 To determine the connection mode, the channel structure of the reactor is figure 1 a, The molar ratio is determined according to the channel volume and the set flow rate, and the heat transfer medium of the heat exchanger is heat transfer oil.
[0027] (2) Mix and stir 150g of anthraquinone and 900g of sulfuric acid to form anthraquinone-sulfuric acid saturated solution; prepare 92.5g of nitric acid, 370g of sulfuric acid and 4.5g of mercury sulfate to make a mixed acid nitration solution of mercury sulfate, and the solvent is dichloroethane. After preheating, the three systems were pumped in respectively through metering pumps, wherein the anthraquinone-sulfuric acid saturated solution was pumped into the micrometer at a flow rate of 14ml / min, the mixed acid nitration solution of mercuric sulfate was 10ml / min, and dichloroethane was pumped at a flow rate of 15ml / min. In the channel reactor, the molar ratio of anthraquinone to nitric acid is 1:2....
Embodiment 2
[0029] refer to figure 2 To determine the connection mode, the channel structure of the reactor is figure 1 b, The molar ratio is determined according to the channel volume and the set flow rate, and the heat transfer medium of the heat exchanger is heat transfer oil.
[0030] 150g of anthraquinone and 600g of sulfuric acid were mixed and stirred to form an anthraquinone-sulfuric acid saturated solution; 92.5g of nitric acid, 370g of sulfuric acid and 7.5g of mercuric sulfate were prepared into a mixed acid nitration solution of mercuric sulfate, and the solvent was dichloroethane. After the three systems were preheated, they were pumped in respectively through metering pumps, wherein the anthraquinone-sulfuric acid saturated solution was pumped into the micrometer at a flow rate of 14ml / min, the mixed acid nitration solution of mercuric sulfate was 14ml / min, and dichloroethane was pumped in at a flow rate of 15ml / min. In the channel reactor, the molar ratio of anthraquinone...
Embodiment 3
[0032] refer to figure 2 To determine the connection mode, the channel structure of the reactor is figure 1 a, The molar ratio is determined according to the channel volume and the set flow rate, and the heat transfer medium of the heat exchanger is heat transfer oil.
[0033] 150g of anthraquinone and 600g of sulfuric acid were mixed and stirred to form an anthraquinone-sulfuric acid saturated solution; 92.5g of nitric acid, 277.5g of sulfuric acid and 7.5g of mercuric sulfate were prepared into a mixed acid nitration solution of mercuric sulfate, and the solvent was dichloroethane. After the three systems were preheated, they were pumped in by metering pumps respectively, wherein the anthraquinone-sulfuric acid saturated solution was pumped into the micrometer at a flow rate of 14ml / min, the mixed acid nitration solution of mercuric sulfate was 15ml / min, and dichloroethane was pumped in at a flow rate of 15ml / min. In the channel reactor, the molar ratio of anthraquinone to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com