Methods of making bempedoic acid and compositions of the same
A kind of compound, technology of ethyl isobutyrate, applied in the field of preparing beipedic acid and composition thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
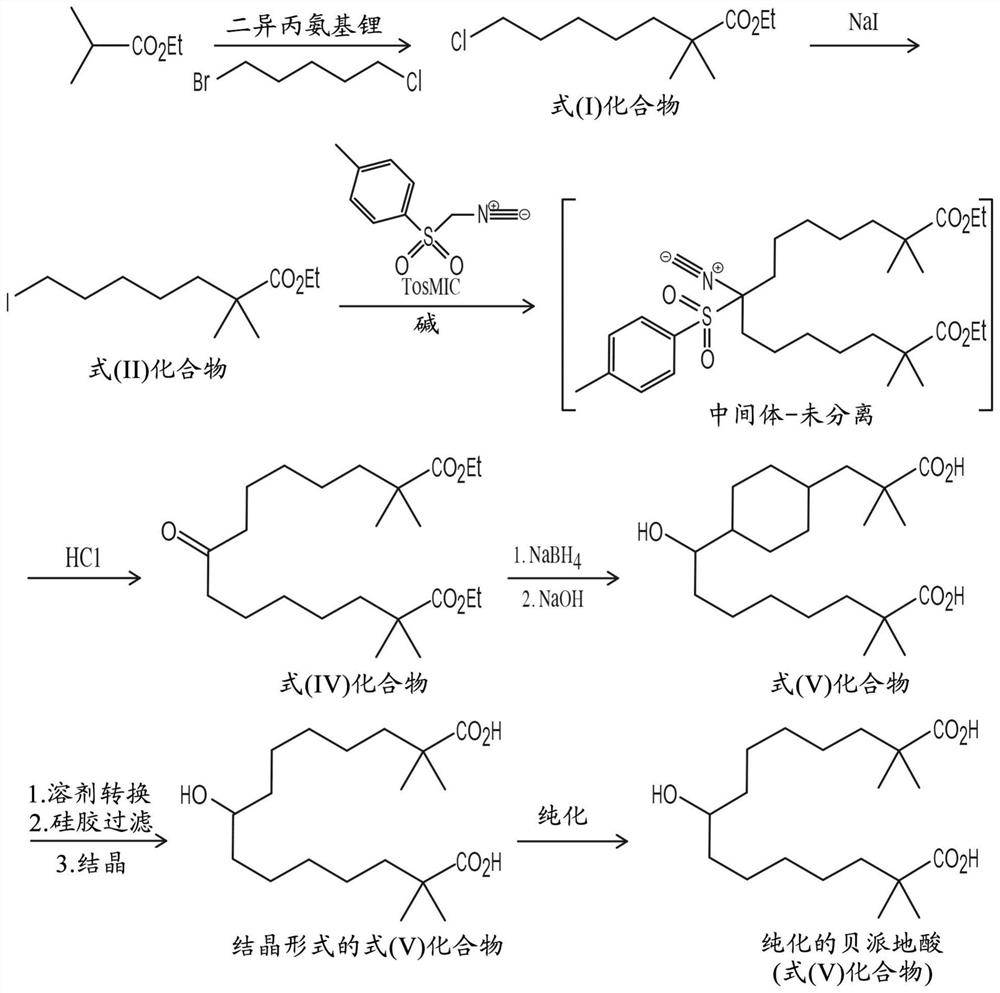
[0560] Embodiment 1: the preparation process of the pharmaceutical material comprising the compound of formula (V) in a purified amount
[0561] In this example, the synthesis of purified bempedelic acid refers to figure 1 .
[0562] Step 1 - Preparation of compound of formula (I)
[0563] Lithium diisopropylamide (LDA) preparation
[0564] Diisopropylamine (317±3 kg, 1.1 equiv) and tetrahydrofuran (THF, 2,102±105 L) were added to the reaction vessel, and the mixture was then cooled to ≤-10°C. n-Butyllithium (n-BuLi, 757±8 kg, 1.2 equivalents) was then added within ≥1 hour while maintaining the temperature ≤-10°C. The feed line was flushed with THF. Addition is highly exothermic. Finally, the batch was cooled back to <-10°C while stirring.
[0565] Alkylation reaction
[0566] Ethyl isobutyrate (317 ± 3 kg, 1.1 equivalents) was added to the reactor at ≤ -10 °C for ≥ 1 hour ( figure 1 ). The batch was stirred while maintaining the temperature < -10°C. 1-Bromo-5-chlo...
Embodiment 2
[0668] Example 2: Alternative Preparation Process for the Preparation of Pharmaceutical Materials Comprising Purified Amounts of Compounds of Formula (V)
[0669] Step 1 - Preparation of compound of formula (I)
[0670] Lithium diisopropylamide (LDA) preparation
[0671] About 321 kg of diisopropylamine and about 1870 L of tetrahydrofuran (THF) were added to the reaction vessel, and the mixture was then cooled to -18°C to -5°C. About 794 kg of n-butyllithium (n-BuLi, solution in heptane) was slowly added while maintaining the temperature at -18°C to -5°C. The batch was maintained at -18°C to -5°C with stirring.
[0672] Alkylation reaction
[0673] Approximately 317 kg of ethyl isobutyrate was added to the reactor containing LDA over a target time of ≥ 1 hour, and the temperature was controlled at -18°C to -5°C. The line is then flushed with about 100 L of THF. The batch was stirred while maintaining the temperature at -18°C to -5°C. Approximately 460 kg of 1-bromo-5-c...
Embodiment 3
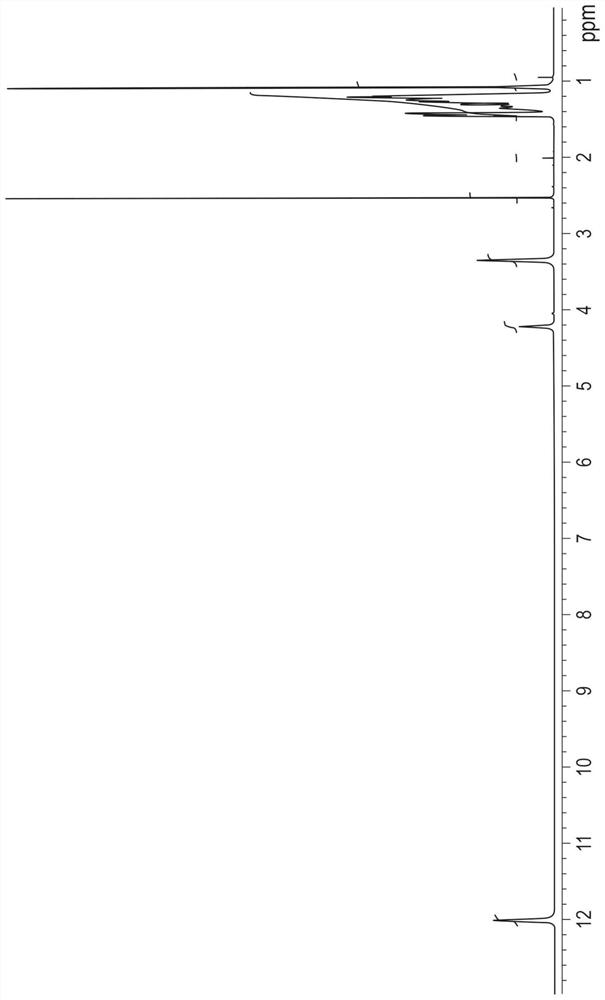
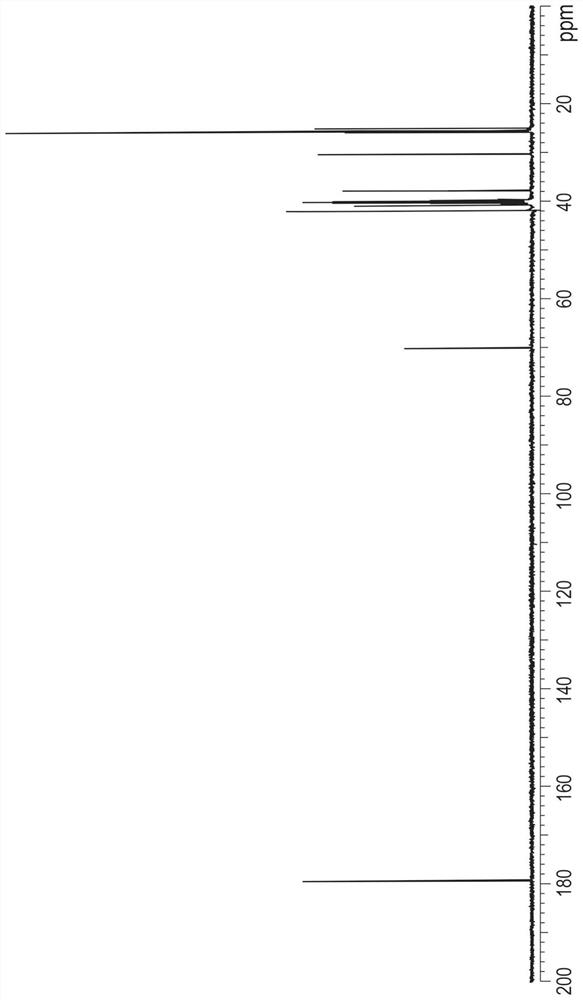
[0734] Embodiment 3: the analytical method of the purity of measuring formula (V) compound
[0735] Determination of impurities
[0736] The impurity content of the compound of formula (V) in purified form was determined using a high performance liquid chromatograph equipped with a gradient function, a thermostatic column compartment and a detector with aerosol detection (CAD).
[0737] The impurity content of the compound of formula (V) in purified form was determined to be 0.05-0.50% w / w.
[0738] Column: Waters XBridge BEH C18 (4.6mm inner diameter x 150mm, 2.5μm)
[0739] Mobile phase: A: 0.05% formic acid (HCOOH) / water (H 2 O)
[0740] Mobile phase: B: 0.05% HCOOH / acetonitrile (ACN)
[0741] Sample temperature: ambient
[0742] Column temperature: 40°C
[0743] Gradient (time: A:B): (0min:90:10; 8.5min., 56:44; 20min., 45:55; 32min., 5:95; 36min., 5:95).
[0744] Flow rate: 1.2mL / min
[0745] Retention time: ~15.2min (bempedic acid in purified form)
[0746] ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com