Asymmetrically substituted phenanthroline-based organic electron transport material as well as preparation and application thereof
A technology for transporting materials and organic electrons, applied in the fields of luminescent materials, organic chemistry, circuits, etc., can solve the problems of low molecular weight, low LUMO, etc., and achieve the effect of improving solubility, low LUMO, high electron injection and transfer electron mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
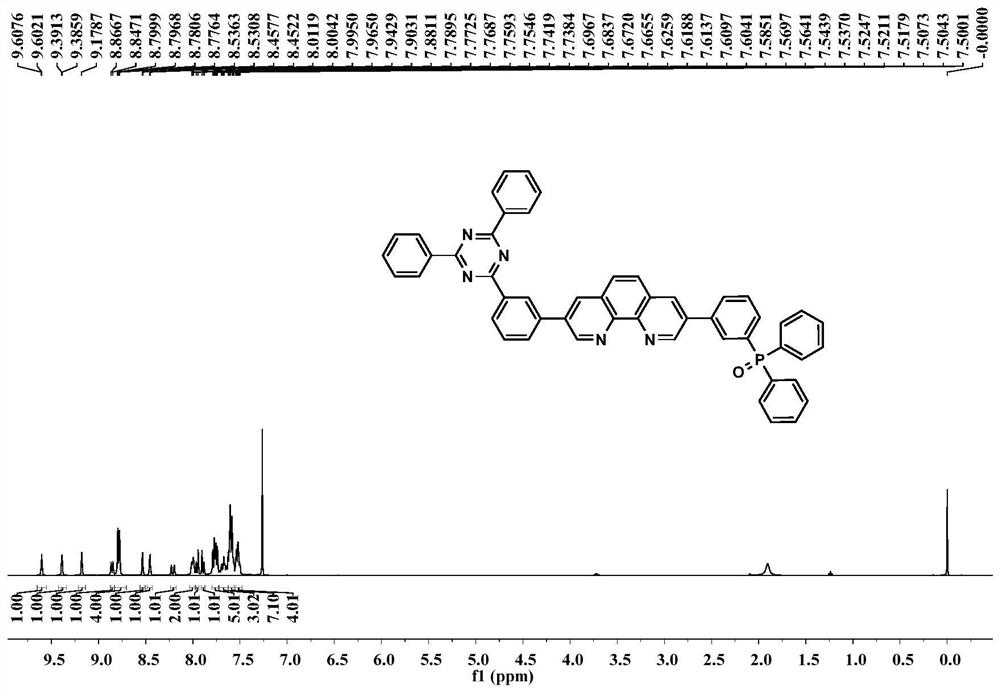
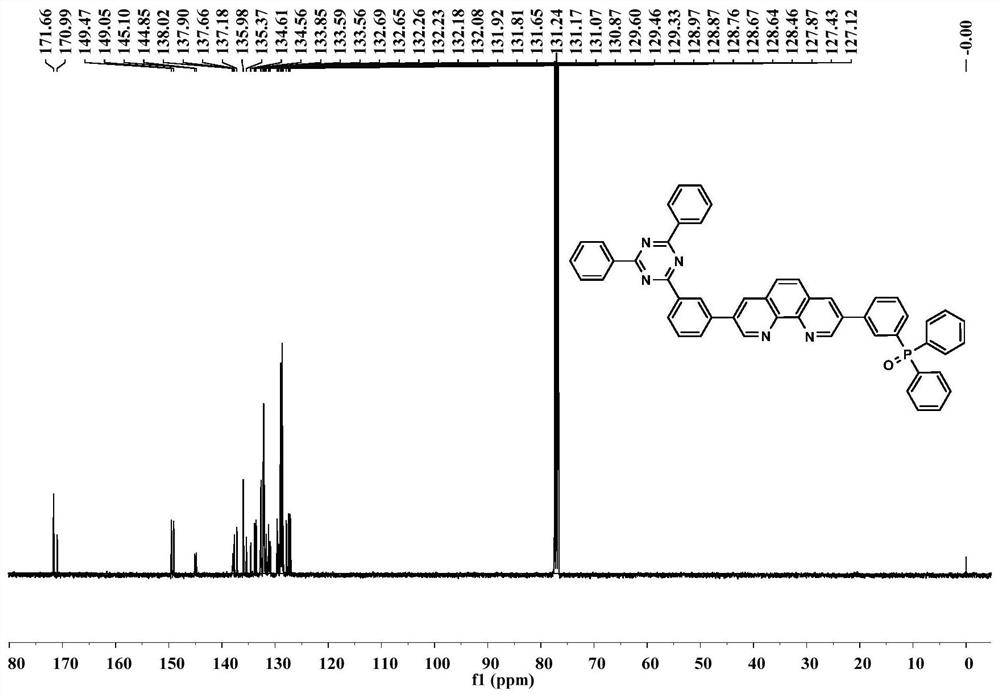
[0034] The structural formula of the organic molecular electron transport material of the present embodiment is as follows:
[0035]
[0036] The preparation method of the organic molecular electron transport material TRZ-Phen-TPO of the present embodiment comprises the following steps:
[0037] Step 1, the preparation of (3-(8-bromo-1,10-phenanthroline-3-yl)phenyl)diphenylphosphine oxide (1)
[0038]
[0039] Under nitrogen atmosphere, the catalyst Pd(OAc) was quickly 2 (45mg, 0.198mmol) and the ligand tricyclohexylphosphine (110mg, 0.396mmol) were added to diphenyl (3-(4,4,5,5-tetramethyl-1,3,2-dioxabor Alkyl-2-yl)phenyl)phosphine oxide (4.0g, 9.89mmol), 3,8-dibromo-1,10-phenanthroline (3.34g, 9.89mmol), Na 2 CO 3In a mixture of aqueous solution (2M, 20mL, 40mmol), ethanol (20mL) and toluene (100mL), the reaction was heated at 90-100°C for 12h, and the reaction progress was confirmed by TLC. After the reaction was completed, it was cooled to room temperature and add...
PUM
| Property | Measurement | Unit |
|---|---|---|
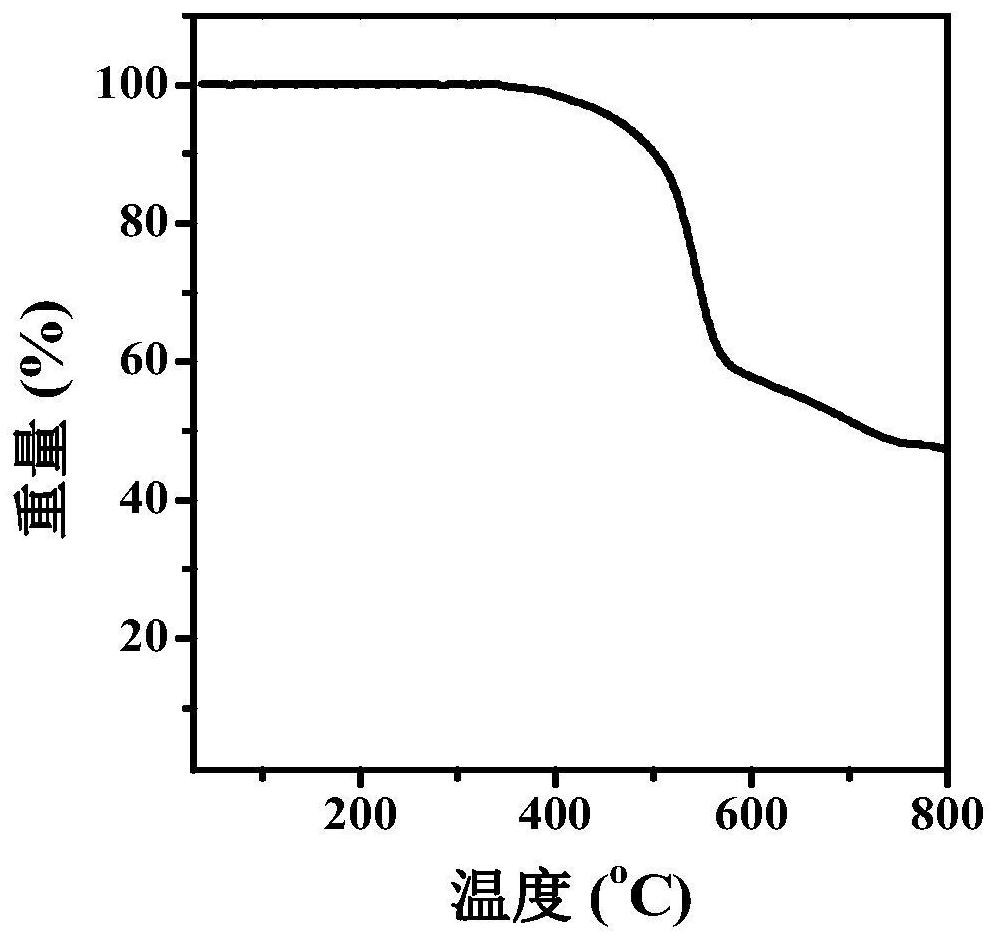
| electron mobility | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com