Thermostable reverse transcriptase
A technology of reverse transcriptase and hydrothermal solution, which is applied to the determination/inspection of transferases, enzymes, microorganisms, etc., and can solve problems such as unsatisfactory thermal stability, demand, and short half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0066] 1. Fusion protein
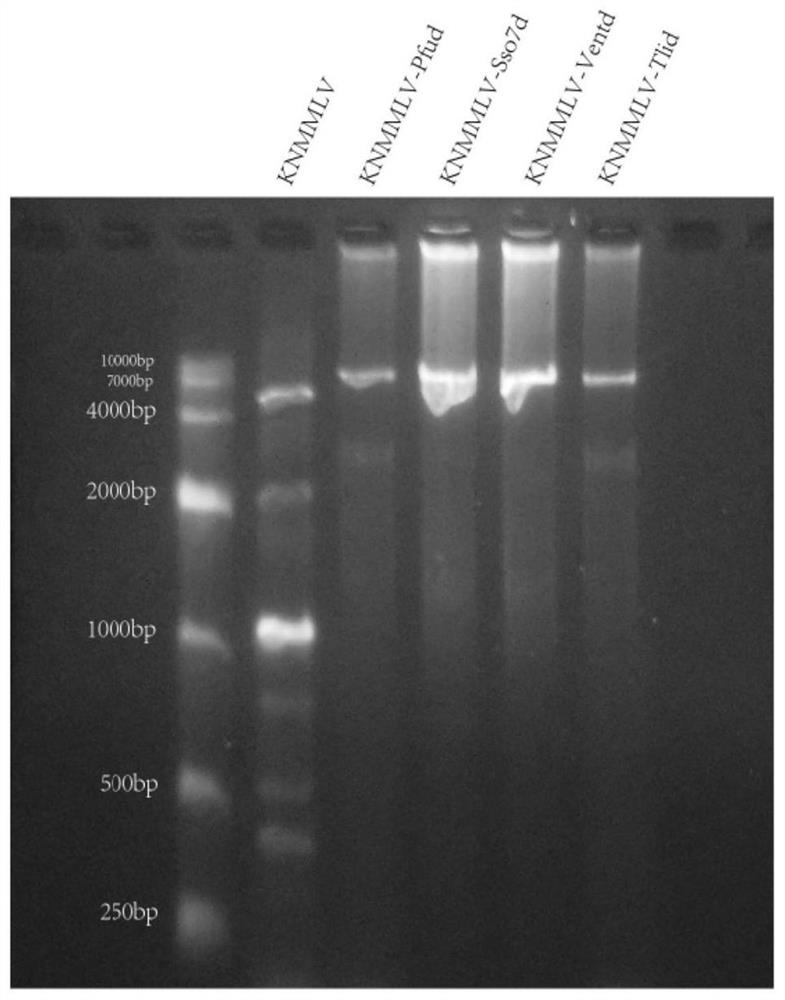
[0067] Taking the following DNA-binding proteins derived from extremophiles or extremophile metagenomes above OTG75°C as examples, MMLV was used as an example to construct:
[0068] The amino acid sequence of the DNA-binding protein Sso7d derived from Saccharolobus solfataricus is shown in SEQID NO:1;
[0069] The amino acid sequence of the DNA binding protein Pfud derived from Pyrococcus furiosus is shown in SEQ ID NO:2;
[0070] The amino acid sequence of the DNA-binding protein Tlid derived from Thermococcus litoralis is shown in SEQ ID NO: 3;
[0071] The amino acid sequence of the DNA binding protein Ventd derived from the hydrothermal vent metagenome is shown in SEQ ID NO:4.
[0072] 2. Fusion of reverse transcriptase and DNA binding protein
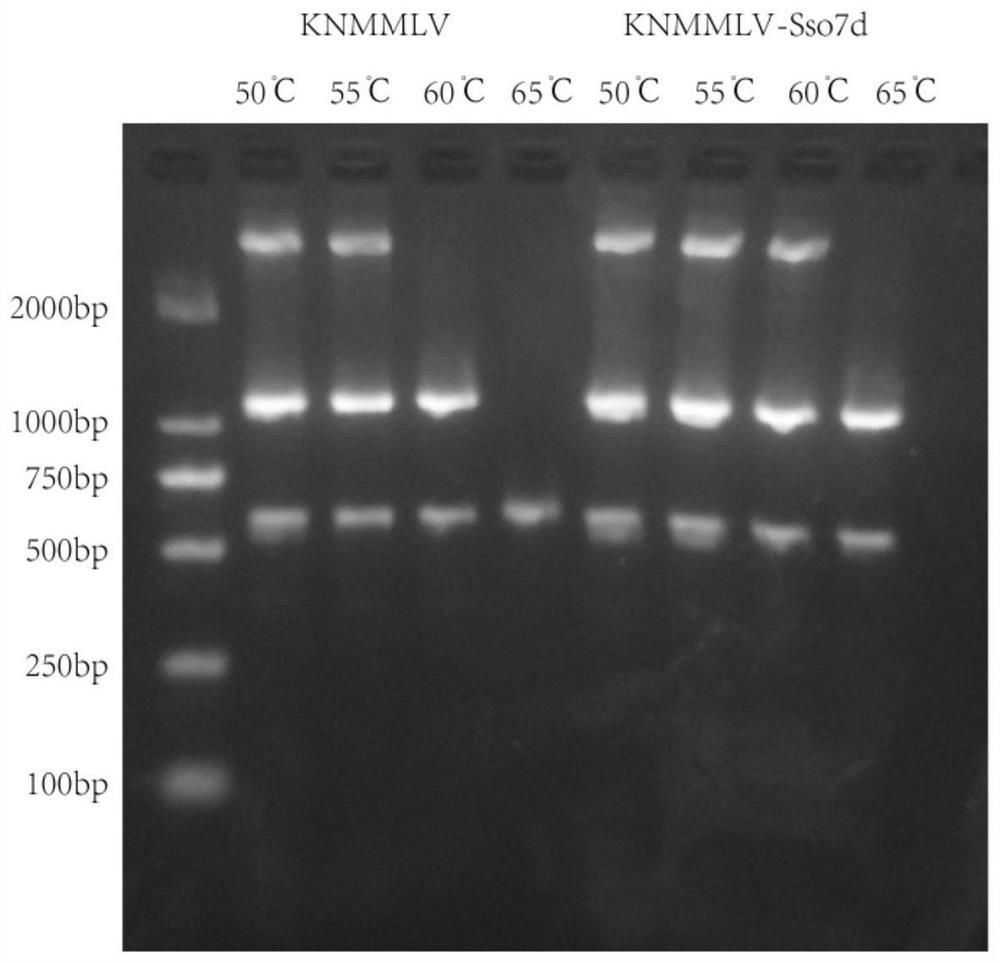
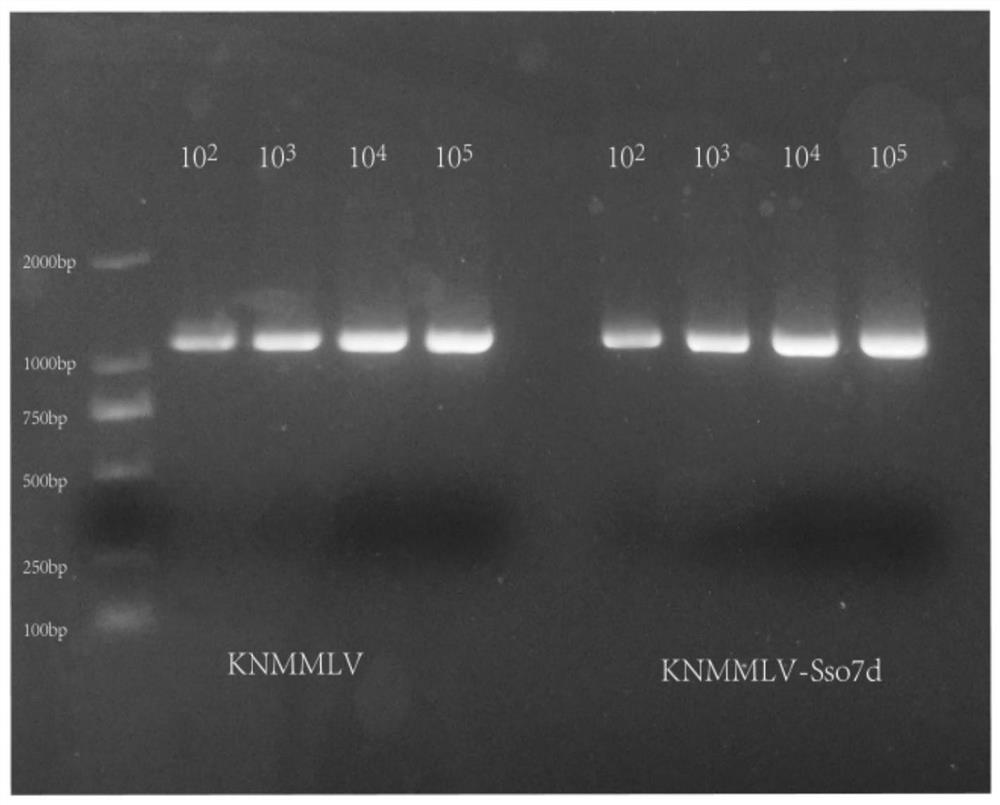
[0073]Fusion of Sso7d at the C-terminus of the MMLV mutant (the amino acid sequence of MMLV is shown in SEQ ID NO: 5, the mutation site L435K / D524N, named KNMMLV after mutation) to obtain KNMMLV-Sso7d ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com