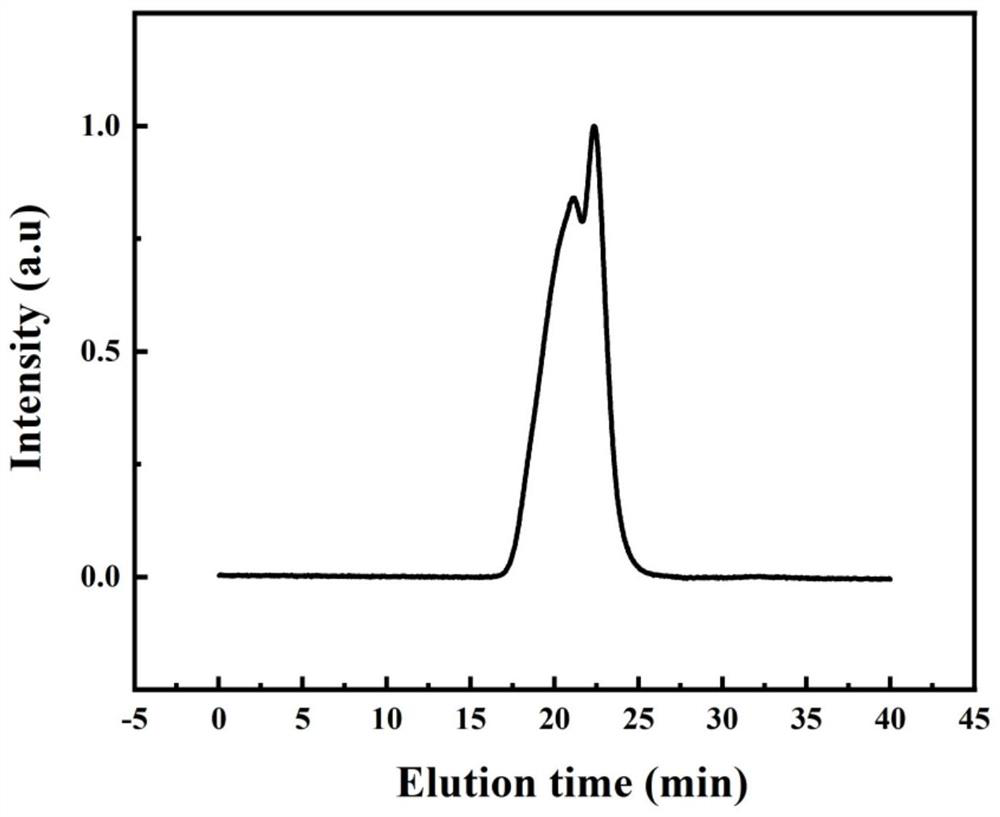
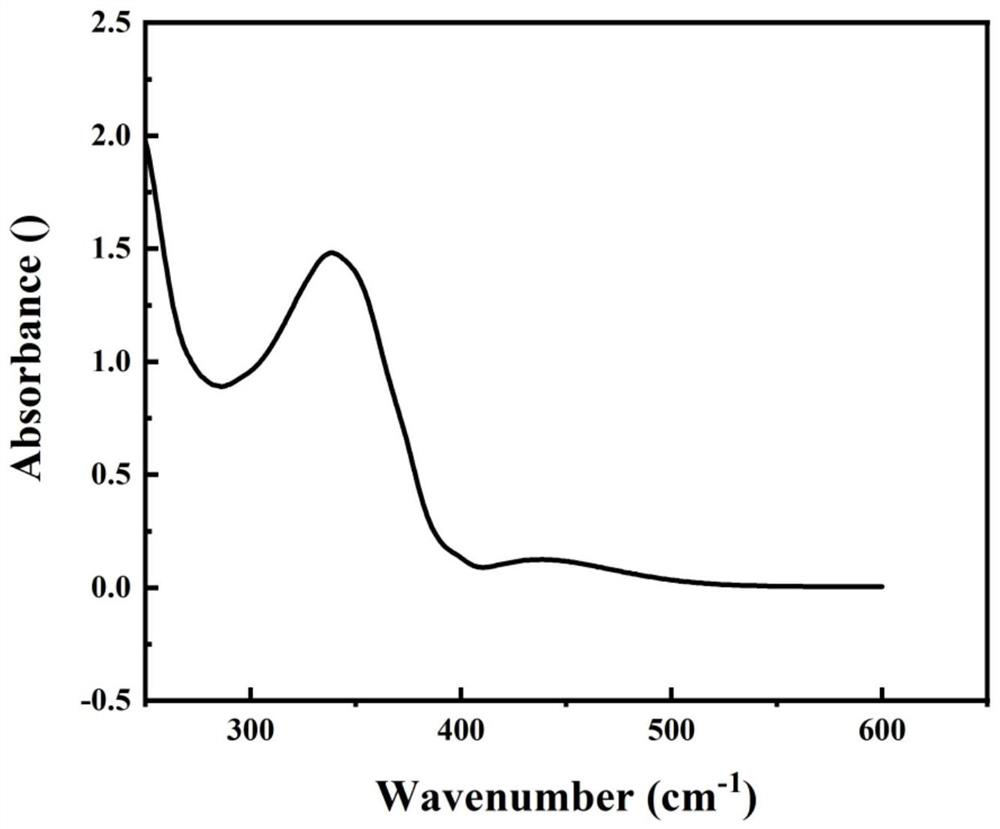
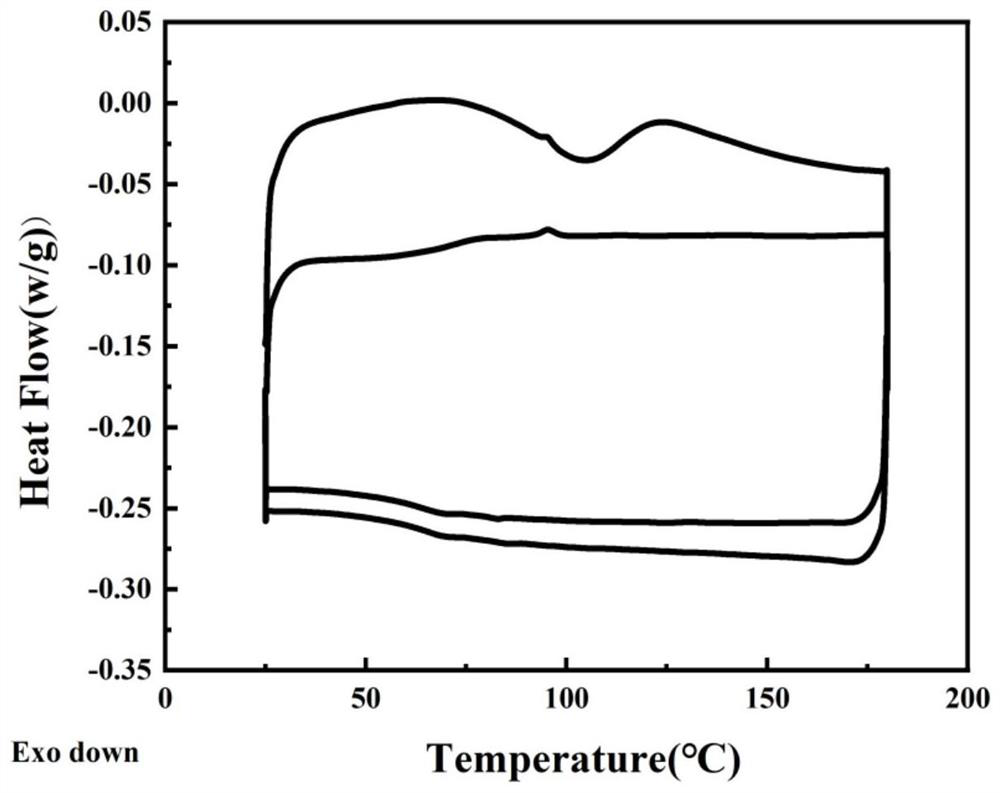
Heterocyclic azobenzene polymer energy storage material and preparation method thereof
A technology of heterocyclic azobenzene and energy storage materials, applied in the field of energy storage materials, can solve problems such as difficult film formation and limited device applications, and achieve the effects of high energy density, mild preparation reaction conditions, and narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A kind of preparation method of heterocyclic azobenzene polymer, its synthetic route is as follows:
[0033]
[0034] 1) Preparation of 4-((3,5-dimethoxyaniline)-diazenyl)-2-thiazole (Azo):
[0035] 2-Aminothiazole (1.0 g, 10 mmol) was dissolved in 10 ml of pyridine, which was then added to 40 mL of NaNO 2 (0.76g, 11mmol) in aqueous solution to obtain a mixed solution. HCl solution (40mL, 1mol·L -1 , 40mmol) was slowly dropped into the above mixed solution for diazotization reaction. After reacting for 0.5h, the reaction solution was slowly added to 3,5-dimethoxyaniline (1.53g, 10mmol) in HCl solution (10ml, 1mol·L -1 ), the whole process was reacted for 6 hours under the protection of argon at a temperature of 0-5°C. A mixed solvent of ethanol and water (50ml, volume ratio 4:1) was added, and reacted at 80°C for 3 hours. The reaction solution was filtered to obtain a crude product, which was purified by column chromatography (dichloromethane / methanol with a vol...
Embodiment 2
[0045] 1) Preparation of 4-((3,5-dimethoxyaniline)-diazenyl)-2-thiazole (Azo):
[0046] 2-Aminothiazole (1.0 g, 10 mmol) was dissolved in 10 ml of pyridine, which was then added to 40 mL of NaNO 2 (0.73g, 10.5mmol) in aqueous solution to obtain a mixed solution. HCl solution (40mL, 1mol·L -1 , 30mmol) was slowly dropped into the above mixed solution for diazotization reaction. After reacting for 0.5h, the reaction solution was slowly added to 3,5-dimethoxyaniline (2.30g, 15mmol) in HCl solution (10ml, 1mol L -1 ), the whole process was reacted for 4 hours under the protection of argon at a temperature of 0-5°C. A mixed solvent of ethanol and water (50ml, volume ratio 4:1) was added and reacted at 70°C for 5 hours. The reaction solution was filtered to obtain a crude product, which was purified by column chromatography (dichloromethane / methanol with a volume ratio of 9:1 as the eluent) to obtain 1.3 g of Azo as a solid.
[0047] 2) Preparation of monomer
[0048] Dissolve c...
Embodiment 3
[0052] 1) Preparation of 4-((3,5-dimethoxyaniline)-diazenyl)-2-thiazole (Azo):
[0053] 2-Aminothiazole (1.0 g, 10 mmol) was dissolved in 10 ml of pyridine, which was then added to 40 mL of NaNO 2 (0.76g, 11mmol) in aqueous solution to obtain a mixed solution. HCl solution (40mL, 1mol·L -1 , 50mmol) was slowly dropped into the above mixed solution for diazotization reaction. After reacting for 0.5h, the reaction solution was slowly added to 3,5-dimethoxyaniline (1.53g, 10mmol) in HCl solution (10ml, 1mol·L -1 ), the whole process was reacted for 10 hours under the protection of argon at a temperature of 0-5°C. A mixed solvent of ethanol and water (50ml, volume ratio 4:1) was added, and reacted at 90°C for 2 hours. The reaction solution was filtered to obtain a crude product, which was purified by column chromatography (dichloromethane / methanol with a volume ratio of 9:1 as the eluent) to obtain 1.4 g of solid Azo.
[0054] 2) Preparation of monomer
[0055] Dissolve cis-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com