Preparation method of catalytic system for olefin hydroformylation
An olefin hydroformylation and catalytic system technology, which is applied in the field of catalytic systems for olefin hydroformylation, can solve the problems of cumbersome preparation process, low positive-to-iso ratio of products, unsuitable for industrialized mass production, etc. Improved stability, satisfying the effect of multiple cycles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
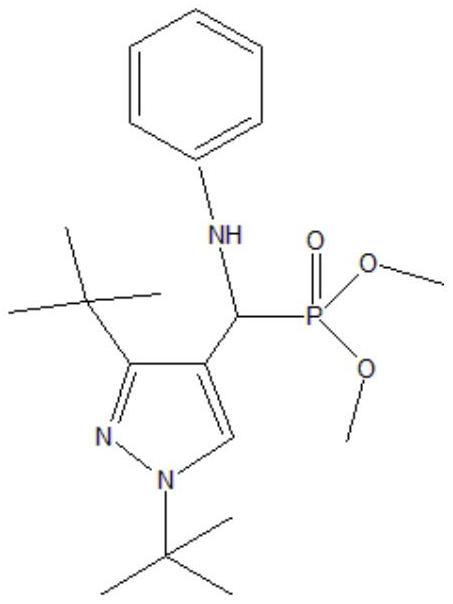
[0027] The selected phosphine ligand is shown in the following formula:
[0028]
[0029] Will Co(acac) 3 (0.001mmol, 0.356mg) and phosphine ligand (0.05mmol, 19.6mg) were dissolved in 10.3mL of toluene, completely dissolved and transferred to a 50mL autoclave. After standing for 1 hour, Rh(acac)(CO) was added 2 (0.01mmol, 2.58mg) and 1-butene (4mmol, 224.44mg), closed reactor, with a certain pressure of synthesis gas (H 2 / CO=1) After replacing the reactor three times, feed synthesis gas until the total pressure is 3MPa. The reactor was heated to 120° C., the reaction temperature was kept constant, and the stirring speed was kept at 500 rpm for 8 hours. After the reaction, take out the reaction kettle and put it in an ice bath, cool the reaction kettle to room temperature or below, carefully take out the reaction product, use GC-MS for qualitative analysis, gas chromatography for quantitative analysis, and after ten cycles, take the solution and use XPS to measure rhodiu...
Embodiment 2
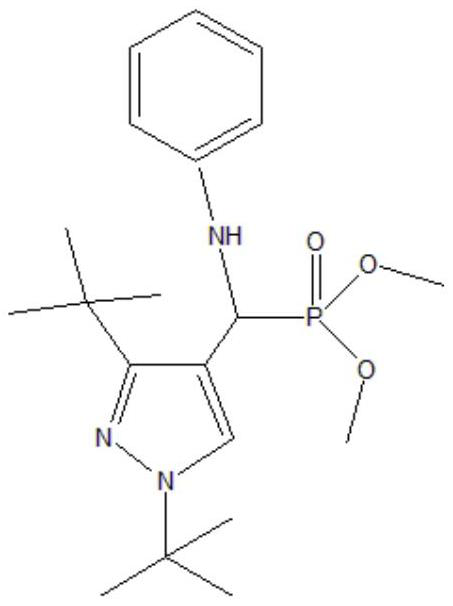
[0032] The selected phosphine ligand is shown in the following formula:
[0033]
[0034] Will Co(acac) 3 (0.002mmol, 0.712mg) and phosphine ligand (0.2mmol, 78.4mg) were dissolved in 5.2mL of toluene and transferred to a 50mL autoclave after complete dissolution. After standing for 1 hour, Rh(acac)(CO) was added 2 (0.01mmol, 2.58mg) and 1-butene (2mmol, 112.22mg), sealed reactor, with a certain pressure of synthesis gas (H 2 / CO=1) After replacing the reactor three times, feed synthesis gas until the total pressure is 1.5 MPa. The reactor was heated to 90° C., the reaction temperature was kept constant, and the stirring speed was kept at 500 rpm for 4 hours. After the reaction, take out the reaction kettle and put it in an ice bath, cool the reaction kettle to room temperature or below, carefully take out the reaction product, use GC-MS for qualitative analysis, gas chromatography for quantitative analysis, and after ten cycles, take the solution and use XPS to measure r...
Embodiment 3
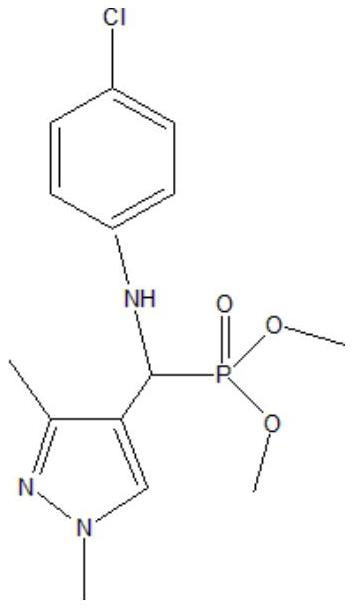
[0037] The selected phosphine ligand is shown in the following formula:
[0038]
[0039] CoCl 3 (0.0015mmol, 0.195mg) and phosphine ligand (0.15mmol, 49.58mg) were dissolved in 1.1mL of toluene, completely dissolved and transferred to a 50mL autoclave. After standing for 1h, RhCl was added 3 (0.01mmol, 2.093mg) and 1-butene (3mmol, 168.33mg), airtight reaction vessel, with certain pressure synthesis gas (H 2 / CO=1) After the reactor was replaced three times, the synthesis gas was introduced until the total pressure was 2MPa. The reactor was heated to 110° C., the reaction temperature was kept constant, and the stirring speed was kept at 500 rpm for 2 hours. After the reaction, take out the reaction kettle and put it in an ice bath, cool the reaction kettle to room temperature or below, carefully take out the reaction product, use GC-MS for qualitative analysis, gas chromatography for quantitative analysis, and after ten cycles, take the solution and use XPS to measure rho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com