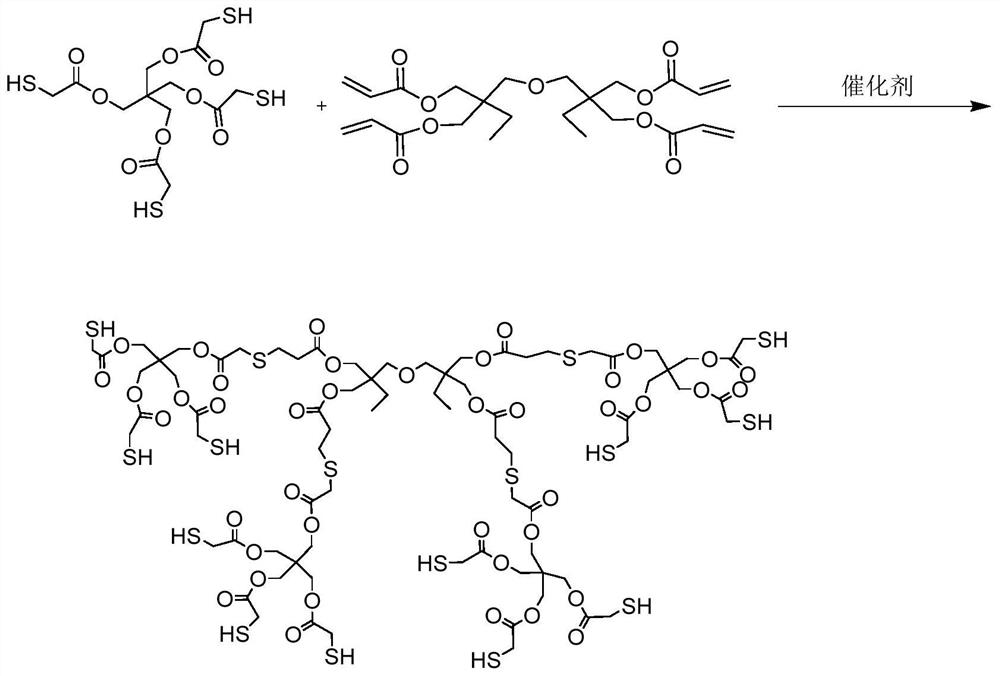
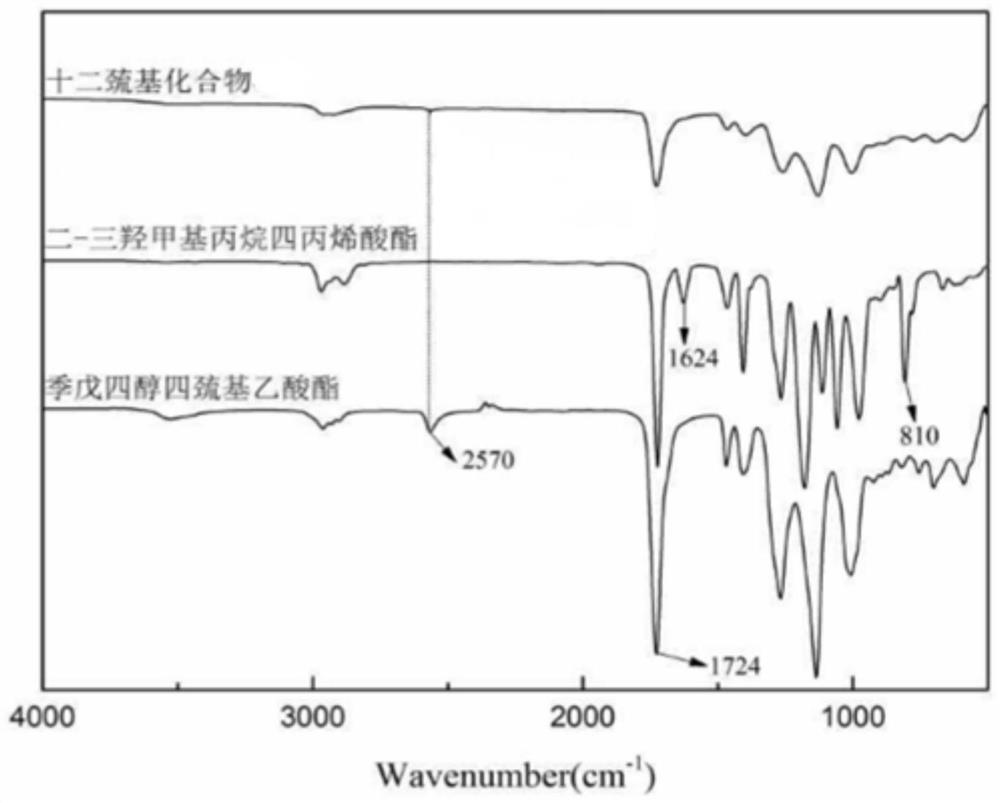
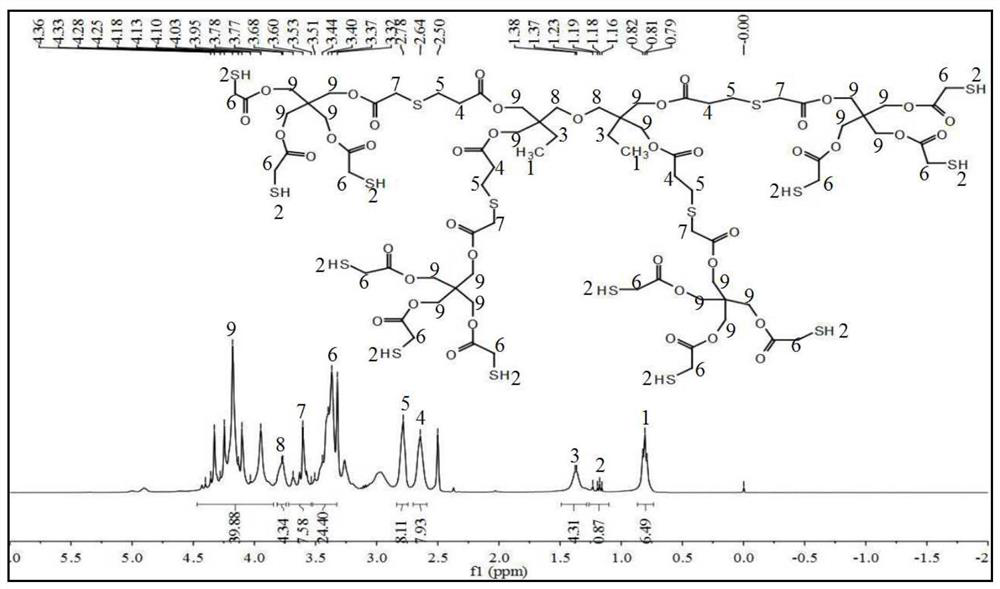
Preparation method of dodecanethiol compound monomer
A technology of mercapto compounds and monomers, which is applied in the field of preparation of dodecyl mercapto compound monomers, can solve the problems of long process route, complicated operation and the like, and achieve the effects of long process route, complicated operation and large pollution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Add 194.67g of parent reactant 0.45mol pentaerythritol tetramercaptoacetate into a 500mL three-necked flask equipped with a condenser, thermometer, water separator, and oil bath, use 300mL ethyl acetate as a solvent, stir mechanically, and heat in an oil bath at 50°C After the pentaerythritol tetramercaptoacetate in the three-necked flask was completely dissolved, 0.35 g of 0.0035 mol of triethylamine catalyst was added dropwise therein, and 35.20 g of 0.1 mol of pentaerythritol tetraacrylate was measured with a graduated cylinder, and then added into the three-necked flask to continue stirring and reacting for 10 h , until TLC determined that the reaction was complete, and the stirring was stopped.
Embodiment 2
[0040] Add 216.3g of parent reactant 0.5mol pentaerythritol tetramercaptoacetate into a 500mL three-neck flask equipped with a condenser, thermometer, water separator, and oil bath, use 300mL ethyl acetate as a solvent, stir mechanically, and heat in an oil bath at 60°C After the pentaerythritol tetramercaptoacetate in the three-necked flask was completely dissolved, 1.06 g of 0.0106 mol of triethylamine catalyst was added dropwise therein, and 35.20 g of 0.1 mol of pentaerythritol tetraacrylate was measured with a graduated cylinder, and then added into the three-necked flask to continue stirring for 12 hours. , until TLC determined that the reaction was complete, and the stirring was stopped.
Embodiment 3
[0042] Add 237.93g of parent reactant 0.55mol pentaerythritol tetramercaptoacetate into a 500mL three-neck flask equipped with a condenser, thermometer, water separator, and oil bath, use 300mL ethyl acetate as a solvent, stir mechanically, and heat in an oil bath at 70°C After the pentaerythritol tetramercaptoacetate in the three-necked flask was completely dissolved, 1.06 g of 0.0106 mol of triethylamine catalyst was added dropwise therein, and then 35.20 g of 0.1 mol of pentaerythritol tetraacrylate was measured with a graduated cylinder, and added into the three-necked flask to continue stirring the reaction 14h, until TLC confirms that the reaction is complete, stop stirring.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com