SiRNA for treating hepatic fibrosis and delivery preparation thereof
A preparation, small interference technology, applied in the direction of DNA/RNA fragments, recombinant DNA technology, medical preparations with non-active ingredients, etc., can solve problems such as adverse reactions, and achieve the effect of reducing expression level and good delivery effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
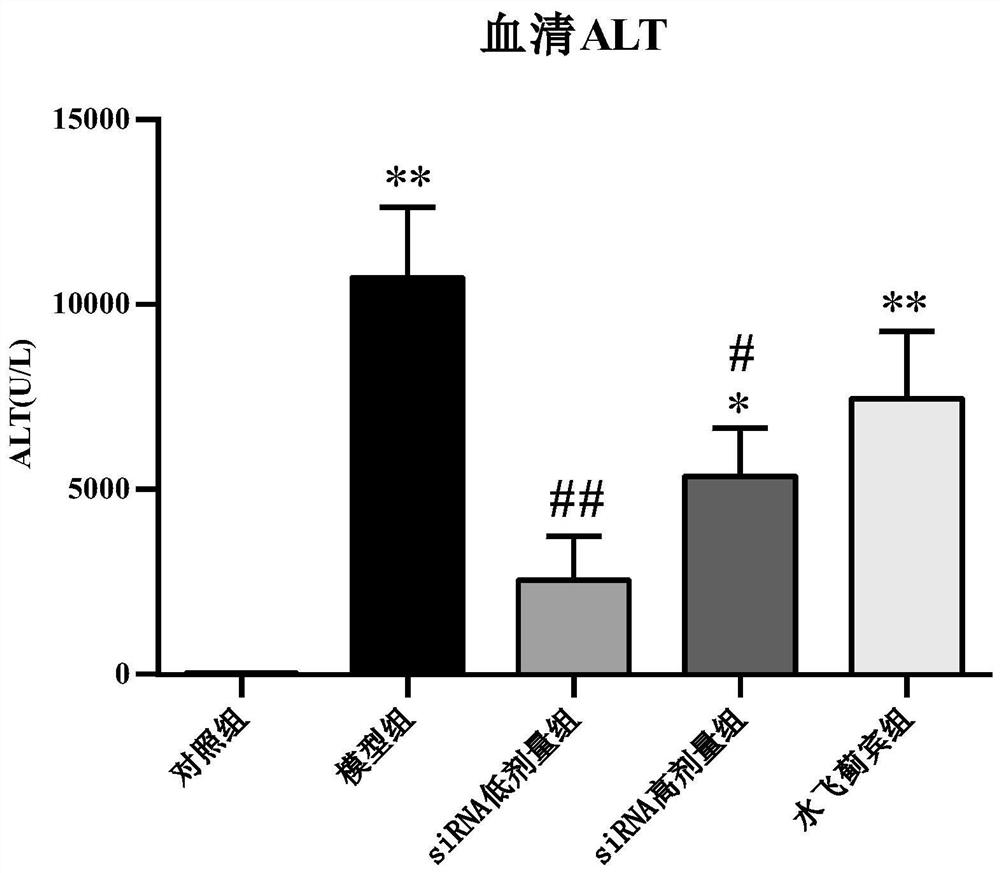
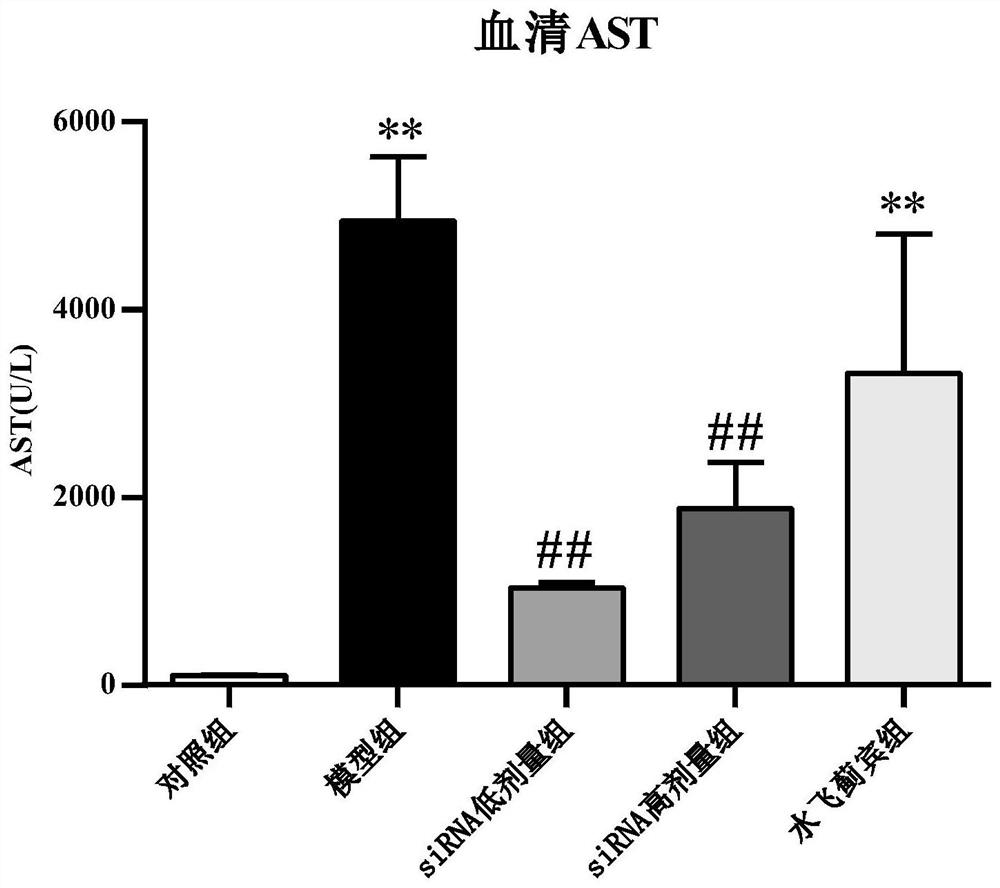
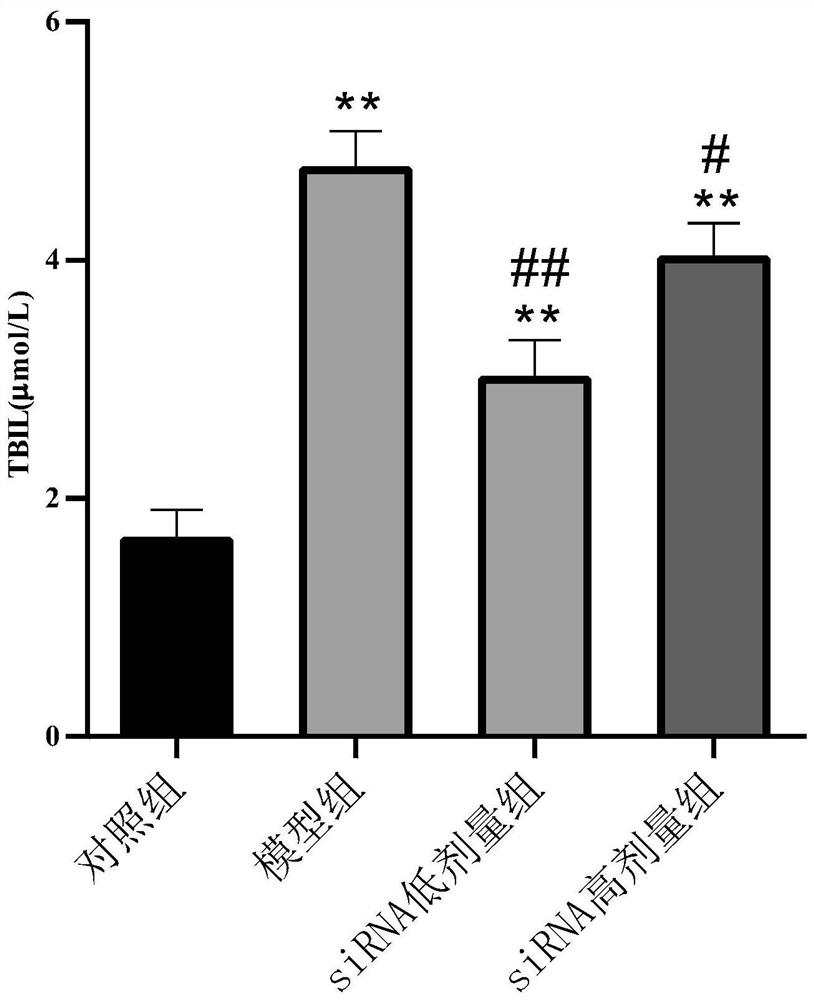
[0035] Example 1: Effect of NOX1 siRNA 1 on carbon tetrachloride-induced acute liver injury in mice, and comparison with positive drug silibinin
[0036] Reagents and Instruments
[0037] CCL4 (purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., article number: C112040, batch: B2027005), soybean oil (Arowana, Q / BBAH0019S), Nox1 siRNA1 (synthesized by Suzhou Beixin Biotechnology Co., Ltd., and the sense chain nucleus Nucleotide sequence: 5'-GAGAUGUGGGAUGAUCGUGACTT-3', antisense strand nucleotide sequence: 5'-GUCACGAUCAUCCCACAUCUCTT-3'), silibinin (purchased from Dalian Meilun Biotechnology Co., Ltd., item number: MB1962, batch : 00801A), CMC-Na (purchased from Beijing Suo Laibao Technology Co., Ltd., article number: C8620, batch: 617O022), in vivo-jetPEI (Polyplus Company of France), paraformaldehyde, liver tissue RNA extraction kit, reverse Transcription Kit.
[0038] experimental method
[0039] There were 56 male C57BL / 6 mice (Victoria), and the weight of th...
Embodiment 2
[0068] Example 2: Effect of NOX1 siRNA 2 on carbon tetrachloride-induced acute liver injury in mice, and comparison with the positive drug silibinin
[0069] Except that the RNA used was Nox1 siRNA 2 (entrusted to Suzhou Beixin Biotechnology Co., Ltd. to synthesize, the sense strand nucleotide sequence: 5'-CAAGCUGGUGGCCUAUAUGAUTT-3', the antisense strand nucleotide sequence: 5'-AUCAUAUAGGCCACCAGCUUGTT-3' ), other operations are the same as in Example 1.
[0070] Obtaining test effect data is equivalent to embodiment 1.
Embodiment 3
[0071] Example 3: Effect of NOX1 siRNA 2 on carbon tetrachloride-induced chronic liver fibrosis model in mice, and comparison with the positive drug silibinin
[0072] Reagents and Instruments
[0073]CCL4 (purchased from Shanghai Aladdin Biochemical Technology Co., Ltd., article number: C112040, batch: B2027005), soybean oil (Arowana, Q / BBAH0019S), Nox1 siRNA 2 (synthesized by Suzhou Beixin Biotechnology Co., Ltd., nucleic acid sequence : 5'-CAAGCUGGUGGCCUAUAUGAUTT-3', antisense nucleic acid sequence: 5'-AUCAUAUAGGCCACCAGCUUGTT-3'), silibinin (purchased from Dalian Meilun Biotechnology Co., Ltd., item number: MB1962, batch: 00801A), CMC- Na (purchased from Beijing Suolaibao Technology Co., Ltd., article number: C8620, batch: 617O022), in vivo-jetPEI, paraformaldehyde, liver tissue RNA extraction kit, reverse transcription kit, intragastric injection needle, syringe, Surgical instruments, inverted microscope, real-time fluorescent quantitative PCR instrument, PCR instrument, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com