Preparation and application of amide compound of ginkgolic acid
A technology of ginkgo phenolic acid amide and amide compound, which is applied in the field of natural product chemistry, can solve the problems of ginkgo toxicity, death, allergic reaction and the like, and achieves the effects of low toxicity, low toxicity, anticancer activity, and low product toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
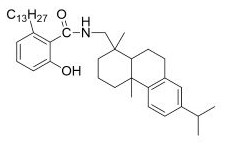
[0025] Preparation method of ginkgolic acid amide compound: using DCC-HoBt condensation method, 0.04 mmol ginkgolic acid (C13:0) and 0.04 mmol HoBt were dissolved in 10 mL ethyl acetate, stirred at 0°C for 30 minutes, and then 0.04 mmol DCC was added. React at 0°C for 2.5 h. Then 2 mL of 0.04 mmol dehydroabietylamine in ethyl acetate solution was added dropwise, the reaction was tracked by TLC, and stirred in an ice bath for 30 minutes, so that DCU was fully separated. DCU was removed by filtration, and after rotary evaporation under reduced pressure, the product was separated and purified by column chromatography (V ethyl acetate: V n-hexane = 1:3), and finally a light brown oil was obtained, 0.014 g, with a yield of 60%.
[0026] Said steps have a molar ratio of ginkgolic acid (C13:0) to dehydroabietylamine of 1:1.0.
[0027] The test results are as follows: m.p. 38-39°C; IR (neat) ν max 3416, 2924, 2852, 1630,1534, 1454, 1375, 1280, 1216, 819, 739, 628; 1H NMR (DMSO- d6...
Embodiment 2
[0030] Preparation method of ginkgolic acid amide compound: using DCC-HoBt condensation method, 0.04 mmol ginkgolic acid (C13:0) and 0.04 mmol HoBt were dissolved in 10 mL ethyl acetate, stirred at 0°C for 30 minutes, and then 0.04 mmol DCC was added. React at 0°C for 2.5 h. Add 2 mL of 0.044 mmol of dehydroabietylamine solution in ethyl acetate dropwise, follow the reaction by TLC, and then stir in an ice bath for 30 minutes, so that DCU is fully separated. DCU was removed by filtration, and the product was separated and purified by column chromatography (V ethyl acetate: V n-hexane = 1:3) after rotary evaporation under reduced pressure, and finally a light brown oil was obtained, 0.015 g, with a yield of 64%.
[0031] The molar ratio of ginkgolic acid (C13:0) to dehydroabietylamine in the described steps is 1:1.1. The test result shows that it is the same as that of Example 1.
Embodiment 3
[0033] Preparation method of ginkgolic acid amide compound: using DCC-HoBt condensation method, 0.04 mmol ginkgolic acid (C13:0) and 0.04 mmol HoBt were dissolved in 10 mL ethyl acetate, stirred at 0°C for 30 minutes, and then 0.04 mmol DCC was added. React at 0°C for 2.5 h. Then 2 mL of 0.048 mmol dehydroabietylamine in ethyl acetate was added dropwise, the reaction was tracked by TLC, and then stirred in an ice bath for 30 minutes, so that DCU was fully separated. DCU was removed by filtration, and after rotary evaporation under reduced pressure, the product was separated and purified by column chromatography (V ethyl acetate: V n-hexane = 1:3), and finally a light brown oil was obtained, 0.014 g, with a yield of 60%.
[0034] Said step is that the molar ratio of ginkgolic acid (C13:0) to dehydroabietylamine is 1:1.2. The test result shows that it is the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com