Pluripotent stem cell, pharmaceutical composition, and preparation method and application thereof
A pluripotent stem cell, stem cell technology, applied in the directions of drug combination, biochemical equipment and method, pharmaceutical formulation, etc., can solve the problems of inability to apply clinically, low induction efficiency, long induction time, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[1303] Preparation Example 1: Preparation of M cells derived from human embryonic stem cells
[1304] 1.1 Generation of embryoid bodies (EBs)
[1305] a. Remove the original culture medium and add PBS to wash;
[1306] b. Use Dispase to digest human embryonic stem cells (Q-CTS-hESC-2, National Stem Cell Resource Bank);
[1307] c. Discard the enzyme solution, add 1ml of KO-DMEM / F12, and draw a vertical well;
[1308] d. Moisten the tip of the gun, suck the liquid in the six-hole plate and transfer it to a 15ml centrifuge tube for centrifugation;
[1309] e. Remove the supernatant from the centrifuged clones, resuspend the cells in a small amount of EB culture medium in a culture dish, add them to a low-attachment culture dish (Corning: Cat. No. 3262), and culture in a 37°C incubator.
[1310] Preparation of EB culture fluid (first medium): add 10% (v / v) of KOSR, 1% (v / v) of NEAA (ie, 0.1mM), 1% (v / v) of KO-DMEM to KO-DMEM ) of GlutaMAX (ie, 2 mM), 8 ng / mL of bFGF, and 0....
Embodiment 1-1
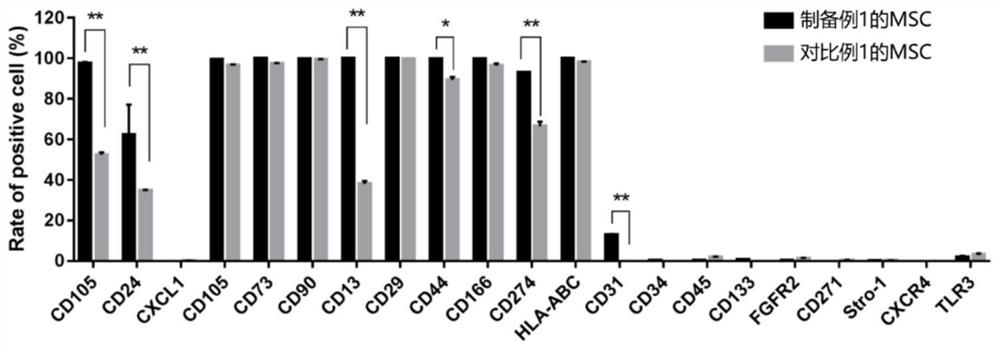
[1379] Example 1-1: Detection of surface markers of M cells
[1380] Detect the expression situation of the M cell surface protein of Preparation Example 1 by flow cytometry:
[1381] 1. Discard the culture supernatant, wash it once with PBS, add Trypsin to digest for 3-5min, and add PBS to stop.
[1382] 2. Collect the cell suspension and centrifuge at 1200rpm for 3min.
[1383] 3. Discard the supernatant, resuspend with PBS, and filter with a cell sieve to remove cell clusters, count, and count as 2×10 per tube. 6 Subpackage.
[1384] 4. Centrifuge at 1200rpm for 3min.
[1385] 5. After blocking with 2% BSA blocking solution for 20 minutes, centrifuge at 1200 rpm for 3 minutes.
[1386] 6. Discard the supernatant, resuspend the cells with 100 μL of 1% BSA antibody diluent, add the direct-labeled antibody, and incubate at room temperature for 30-45 minutes.
[1387] 7. Wash three times with 1mL PBS, centrifuge at 1200rpm for 3min, and discard the supernatant.
[1388] 8...
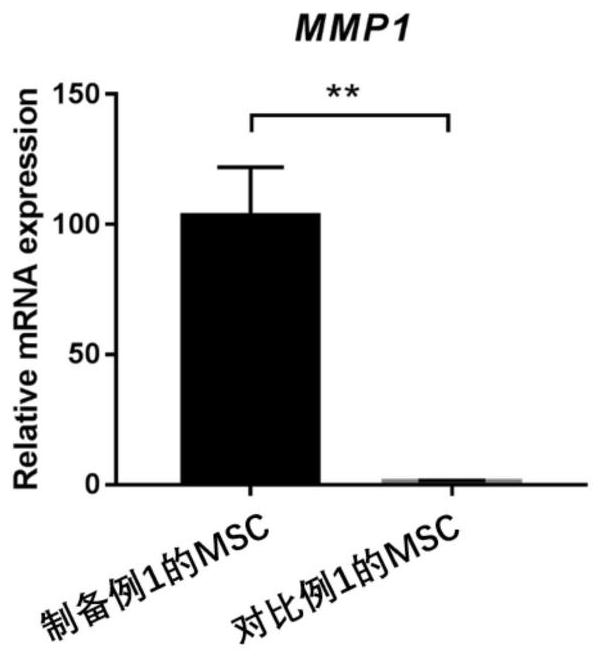
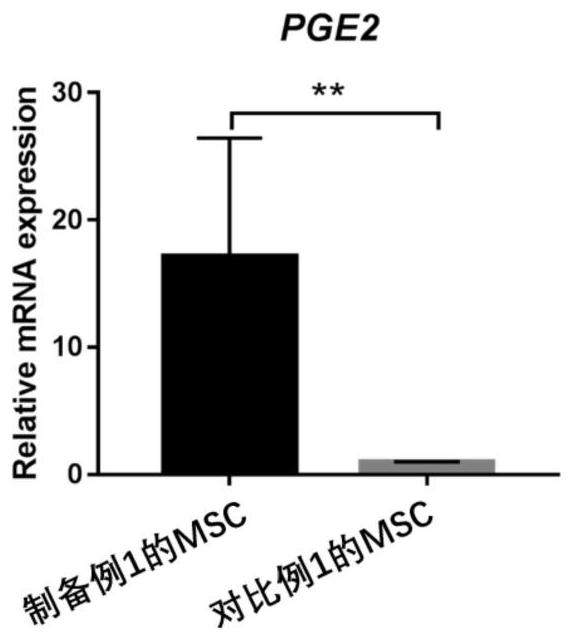
Embodiment 1-2
[1392] Example 1-2: Detection of cytokine expression levels in M cells
[1393] The expression levels of cytokines in the M cells of Preparation Example 1 were measured by Real-time PCR.
[1394] 1. Extraction of cellular RNA
[1395] Use the RNA extraction kit to extract, the specific steps are as follows:
[1396] (1) Add 10 μL of β-mercaptoethanol to each ml of RL lysate, and lyse the cells on ice according to the amount of cells;
[1397] (2) Transfer the lysed liquid to the CS column, centrifuge at 12000rpm for 2min;
[1398] (3) Add 1 volume of 70% ethanol to the filtrate, mix well and transfer to CR3, centrifuge at 12000rpm for 1min;
[1399] (4) Pour off the filtrate, add 350 μL RW1 to CR3, centrifuge at 12000 rpm for 1 min;
[1400] (5) Pour off the filtrate, add 80 μL DNase I working solution to CR3, room temperature for 15 minutes;
[1401] (6) Add 350 μL RW1 to CR3, centrifuge at 12000 rpm for 1 min;
[1402] (7) Pour off the filtrate, add 500 μL RW to CR3,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com