A kind of preparation method of optically active menthol
An optically active, menthol technology, applied in directions such as organic chemistry methods, chemical instruments and methods, and the preparation of organic compounds, can solve problems such as low L-menthol yield, complex resolution steps, etc. The effect of novel route and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
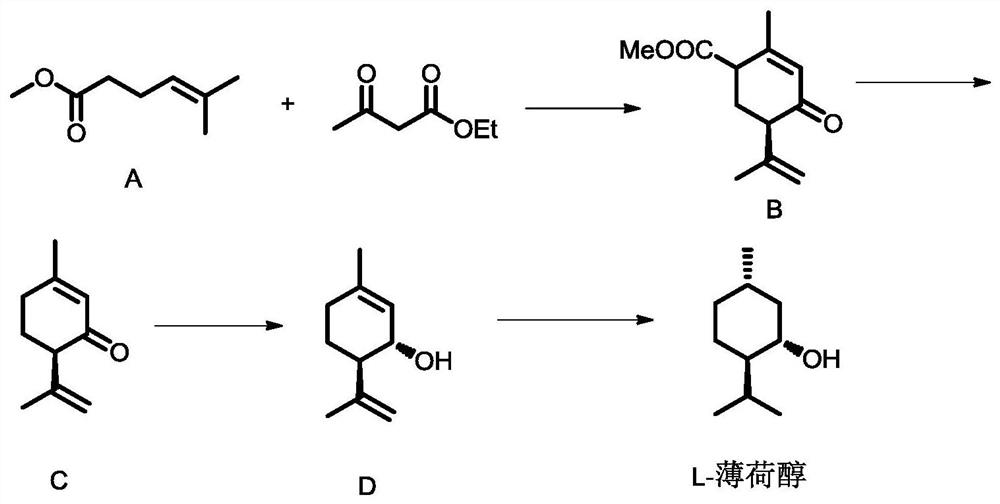
[0041] Preparation of Compound B
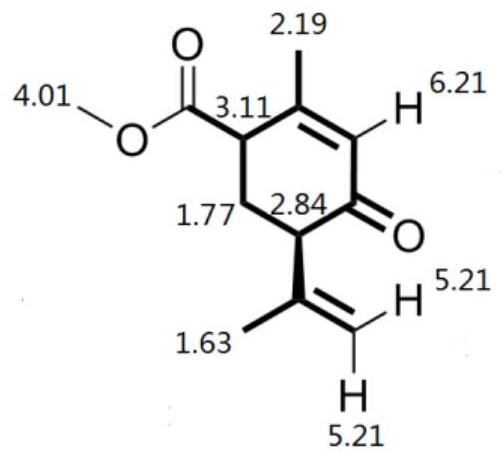
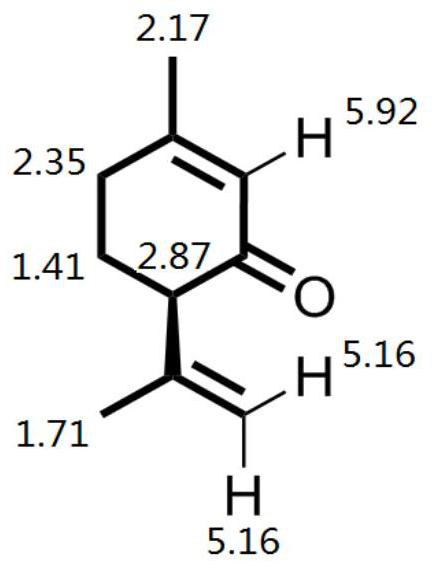
[0042] Take compound A 71g, Na 2 CO 3 0.53g was added to the 250ml reaction kettle, and after stirring for 25min at 20°C, 46g of ethyl acetoacetate was added, CuSO 4 ·H 2 O chiral phosphine d catalyst 0.117g, the reaction system was heated to 100°C, and the reaction was kept for 9h. The conversion rate of compound A was determined to be 99% by gas chromatography, and the product was optically active compound B with an optical purity of 95ee%. The catalyst in the reaction solution was removed by filtration while it was hot, the filtrate was transferred to a crystallization kettle, 50 g of ethyl acetate was added, the temperature was lowered by program from 90 ° C to 20 ° C, and the temperature was lowered by 1 ° C every 5 minutes, and the compound B was obtained by insulation filtration. White crystals with optical purity It is 99.9ee%, and the single-pass crystallization yield is 95%; the mother liquor is applied mechanically, and the to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| crystallization temperature | aaaaa | aaaaa |
| crystallization temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com