Quinazolinedione compound, preparation method and application thereof, and herbicide
A quinazoline dione and compound technology, which is applied in the field of pesticide herbicides, can solve the problems of lack of HPPD-inhibiting herbicides and the like, and achieve the effects of high safety and good control effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
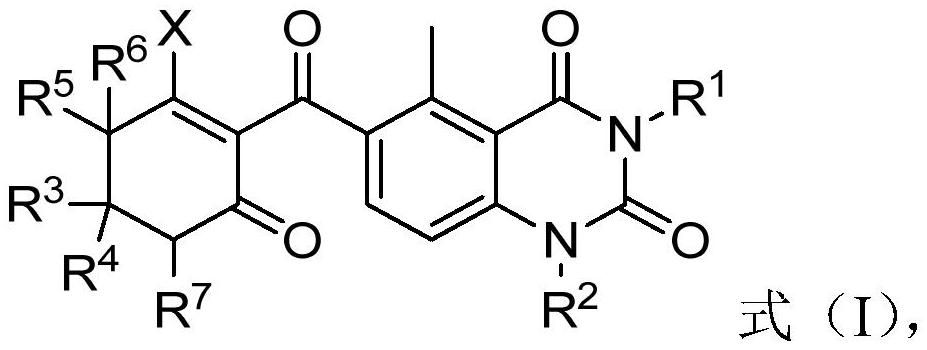
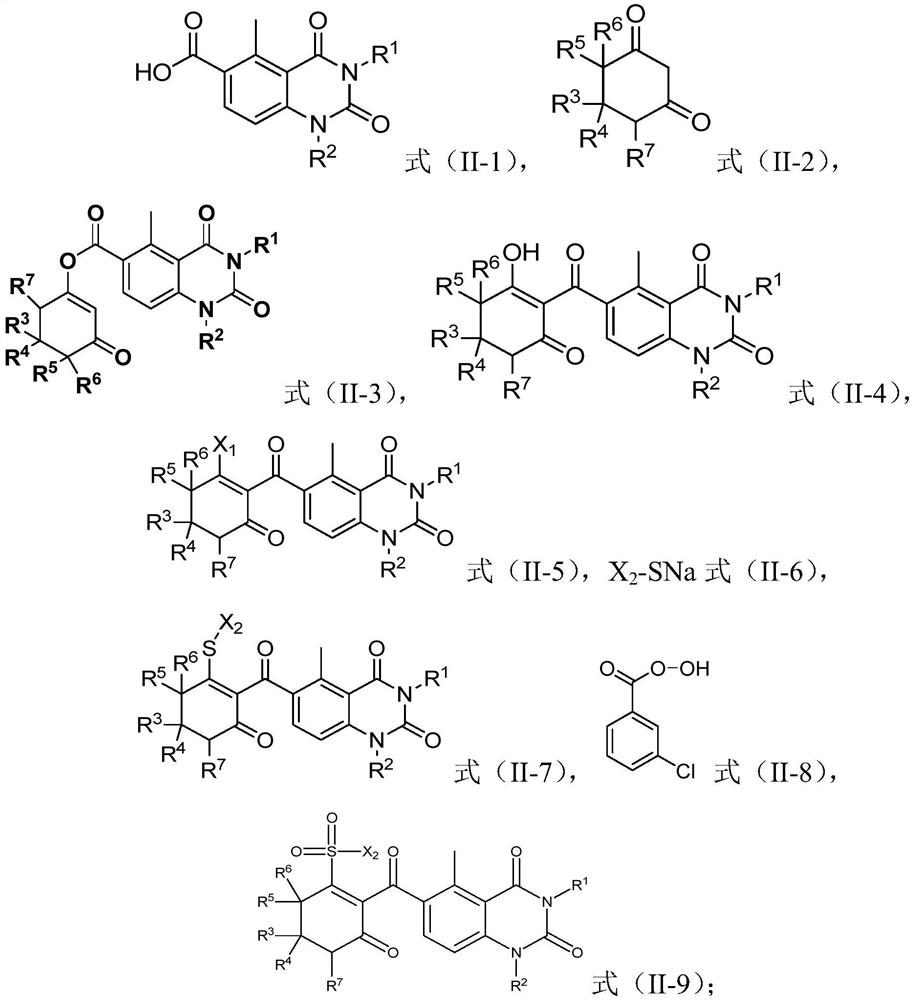
[0095] According to the method of preparing a quinazoline diketone compound according to the preparation of the formula (I), the structure of the formula (II-3) can be made according to the conventional conditions and operation of the rearrangement reaction. The compound is in contact with the catalyst in the presence of a base and solvent.
[0096]Preferably, the compound of the formula (II-3) is 1: (0.5-4): (0.5-4): (0.5-4): (0.5-4): (0.5-4): (II-3) The compound and the catalyst and the alkali have a molar ratio of 1: (0.05-1): (1-3).
[0097] Preferably, in step (2), the condition comprising: the reaction temperature is 0-100 ° C; the reaction time is 0.5-36h; more preferably, in step (2), the condition of the contact comprises : The reaction temperature was 20-40 ° C; the reaction time was 5-30 h.
[0098] It will be understood by those skilled in the art that the methods of the present invention may also include the step of purifying the resulting product, and there is no par...
preparation example 1
[0121] Preparation Example 1: Preparation of Compounds of Structure shown in Formula (II-4-1)
[0122]
[0123] At room temperature, 75.5 g of a compound of A was added to a 1L reaction bottle, and 300 ml of ice acetic acid was added with stirring. After the dropwise addition, the stirring was continued for about 2.5 h. After the reaction was completed, the reaction solution was filtered, and the resulting solid was washed with acetonitrile 200ml and 200 ml of ice acetate, and the intermediate B was obtained, and the yield was 95%; melting point: 186-188 ° C. 1 H NMR (600MHz, DMSO-D 6 : Δ8.97 (BRS, 3H), 7.72 (D, J = 8.4 Hz, 1H), 6.75 (D, J = 7.8 Hz, 1H), 2.40 (s, 3h).
[0124] 8.31 g of intermediate B was added to 200 ml of two-neck cylinders, 80 ml of pyridine, and 2.55 g of C-1 N-n-1 is added slowly to the system with stirring. The reaction liquid was heated to 100 ° C for overnight. After the reaction was completed, the pyridine was distilled under reduced pressure, and the r...
preparation example 2
[0130] Preparation Example 2: Preparation of Compound 2
[0131]
[0132] The compounds of 0.925 g of II-4-1 were added to 50 ml of two-necked cylinders, and 15 ml of anhydrous methylene chloride was added, and 0.945 g of ohloyl chloride was added to the system. The reaction at room temperature for 15 h, TLC tracked to the reaction raw material disappeared. After the reaction was completed, the organic phase was washed three times with saturated sodium bicarbonate, combined with an organic layer, and dried over anhydrous sodium sulfate. The dissociation was removed from the solvent to give a white solid, ie compound 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com