Preparation method of tofacitinib hydrolysis impurity
A technology for tofacitinib and impurities, which is applied in the field of drug synthesis, can solve the problems of complicated operation, difficult separation of two hydrolyzed impurities, low yield and the like, and achieves the effects of simple operation steps, short route and high reaction yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
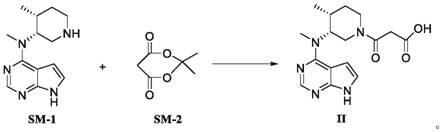
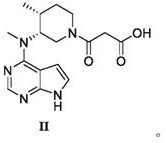
[0030] Dissolve cyclo(ethylene)isopropyl malonate (17.28g, 0.12mol) in 50ml of acetonitrile, add N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl) -7H-Pyrrolo[2,3-d]pyrimidin-4-amine (24.53g, 0.10mol), stirred at room temperature until the reaction solution dropped to 0°C after the reaction was completed, and crystallized by adding 20ml of methyl tert-butyl ether to obtain Tofacitinib hydrolyzed the impurities, the yield was 87.7%, and the HPLC purity was 99.95%.
Embodiment 2
[0032] Dissolve cyclo(ethylene)isopropyl malonate (17.28g, 0.12mol) in 50ml DMF, add N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl)- 7H-Pyrrolo[2,3-d]pyrimidin-4-amine (24.53g, 0.10mol), stirred at room temperature until the reaction solution dropped to 5°C after the reaction, added 30ml of acetone for crystallization to obtain tofacitinib hydrolyzed impurities , the yield was 85.5%, and the pure HPLC degree was 99.92%.
Embodiment 3
[0034] Dissolve cyclo(ethylene)isopropyl malonate (17.28g, 0.12mol) in 50ml toluene, add N-methyl-N-((3R,4R)-4-methylpiperidin-3-yl) -7H-Pyrrolo[2,3-d]pyrimidin-4-amine (24.53g, 0.10mol), stirred at room temperature until the reaction solution dropped to 8°C after the end of the reaction, added 25ml isopropyl ether for crystallization to obtain tofacitinib The impurities were hydrolyzed, the yield was 84.1%, and the HPLC purity was 99.81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com