Polypeptide microneedle and preparation method thereof
A technology of microneedles and needle bodies, which is applied in the direction of microneedles, needles, and medical formulas, and achieves the effects of simple preparation method, eye bag removal, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Example 1 Preparation of anti-wrinkle and anti-aging polypeptide microneedles
[0029] 1.1 Preparation of needle solution
[0030] According to the mass percentage, 85% hyaluronic acid, 0.01% acetyl hexapeptide-8, 0.005% β-alanyl hydroxyprolyl diaminobutyryl benzylamide, and the rest of water are fully stirred and mixed Dissolved and vacuum degassed to obtain a needle solution.
[0031] 1.2 Preparation of pedestal solution
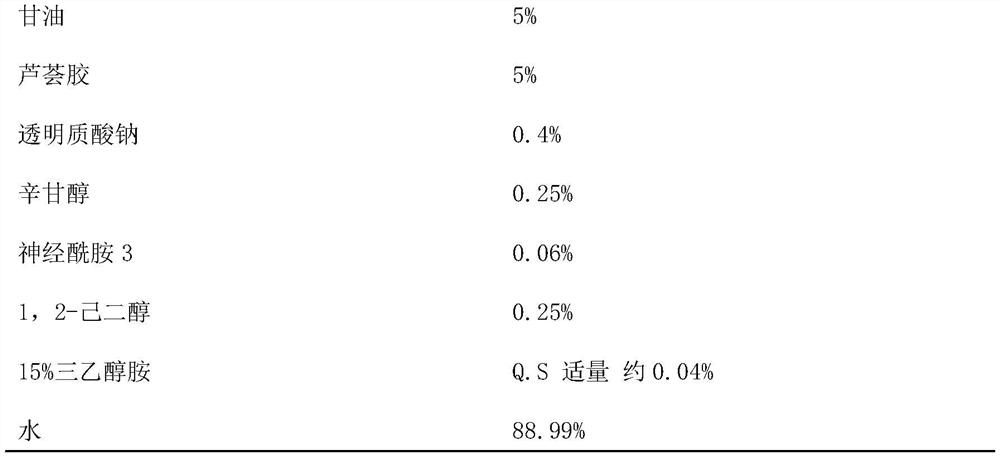
[0032] According to the mass percentage, hyaluronic acid is added into water, stirred and dissolved to obtain a solution with a mass fraction of 1.5%, and a base solution is obtained after vacuum defoaming treatment.
[0033] 1.3 Preparation of anti-wrinkle and anti-aging polypeptide microneedles
[0034] Pour the needle solution into the female mold. After the needle solution fills the pinholes of the mold, cover the base solution on the mold containing the needle solution, and dry it at a temperature of 25°C and a humidity of 30%. 10h, after d...
Embodiment 2
[0035] Example 2 Preparation of Acne Removing and Repairing Polypeptide Microneedles
[0036] 2.1 Preparation of needle solution
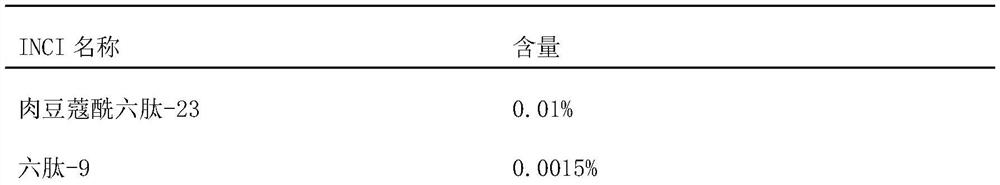
[0037] According to the mass percentage, 85% hyaluronic acid, 0.01% myristoyl hexapeptide-23, 0.0015% hexapeptide-9, and the rest of water are fully stirred and dissolved, and the needle body solution is obtained after vacuum defoaming treatment .
[0038] 2.2 Preparation of pedestal solution
[0039] According to the mass percentage, hyaluronic acid is added into water, stirred and dissolved to obtain a solution with a mass fraction of 1.5%, and a base solution is obtained after vacuum defoaming treatment.
[0040] 2.3 Preparation of acne repairing polypeptide microneedles
[0041] Pour the needle solution into the female mold. After the needle solution fills the pinholes of the mold, cover the base solution on the mold containing the needle solution, and dry it at a temperature of 25°C and a humidity of 30%. 10h, after demoulding, it is ready...
Embodiment 3
[0042] Example 3 Preparation of Salicylic Acid Antimicrobial Peptide Microneedles
[0043] 3.1 Preparation of needle solution
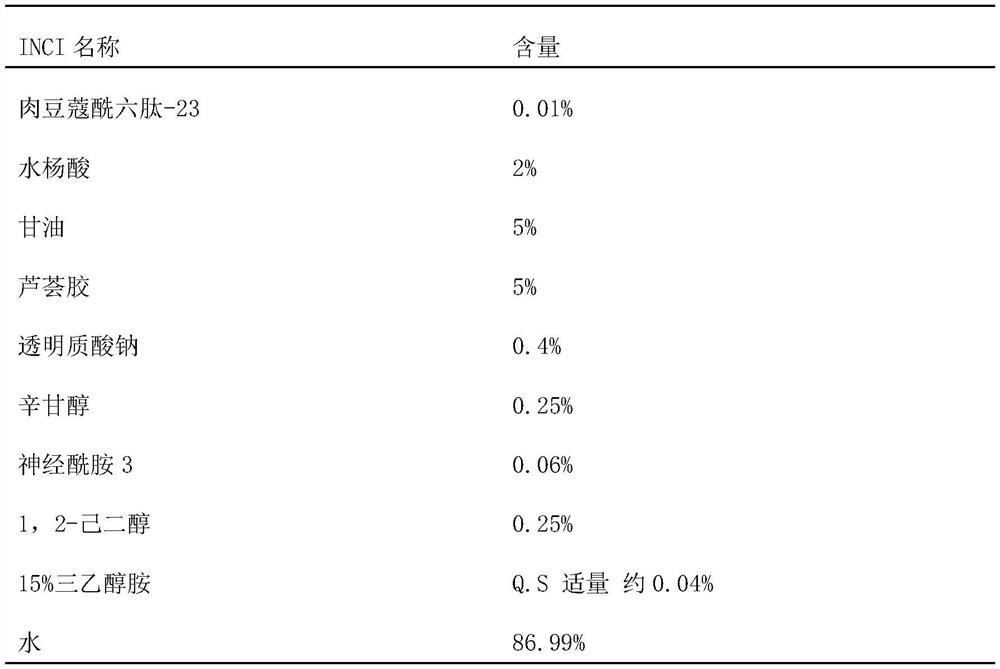
[0044] According to the mass percentage, 80% hyaluronic acid, 0.01% myristoyl hexapeptide-23, 2% salicylic acid, and the rest of water are fully stirred, mixed and dissolved, and the needle body solution is obtained after vacuum defoaming treatment.
[0045]3.2 Preparation of pedestal solution
[0046] According to the mass percentage, hyaluronic acid is added into water, stirred and dissolved to obtain a solution with a mass fraction of 1.5%, and a base solution is obtained after vacuum defoaming treatment.
[0047] 3.3 Preparation of salicylic acid antimicrobial peptide microneedles
[0048] Pour the needle solution into the female mold. After the needle solution fills the pinholes of the mold, cover the base solution on the mold containing the needle solution, and dry it at a temperature of 25°C and a humidity of 30%. 10h, after demoulding, it i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com