Tetraborate intermediate for photoelectric material synthesis and preparation method of tetraborate intermediate
A technology of tetraborate and optoelectronic materials, which is applied in chemical instruments and methods, compounds containing elements of Group 3/13 of the periodic table, organic chemistry, etc., and can solve the problems of complex steps and long synthetic routes of macromolecules.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0032] Example 1: Preparation of spirofluorene tetraborate
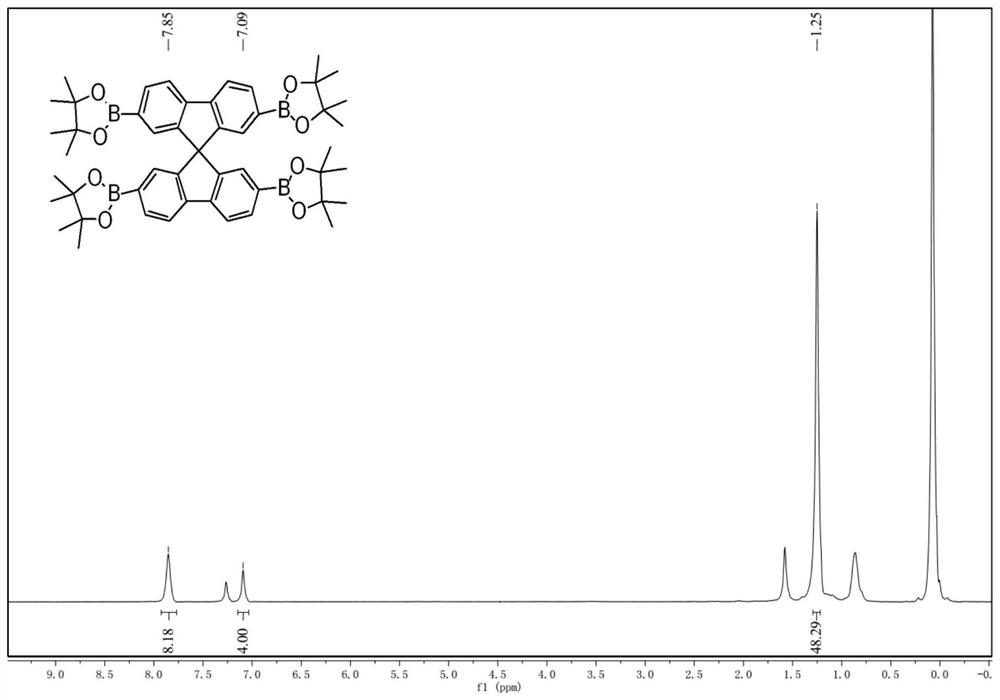
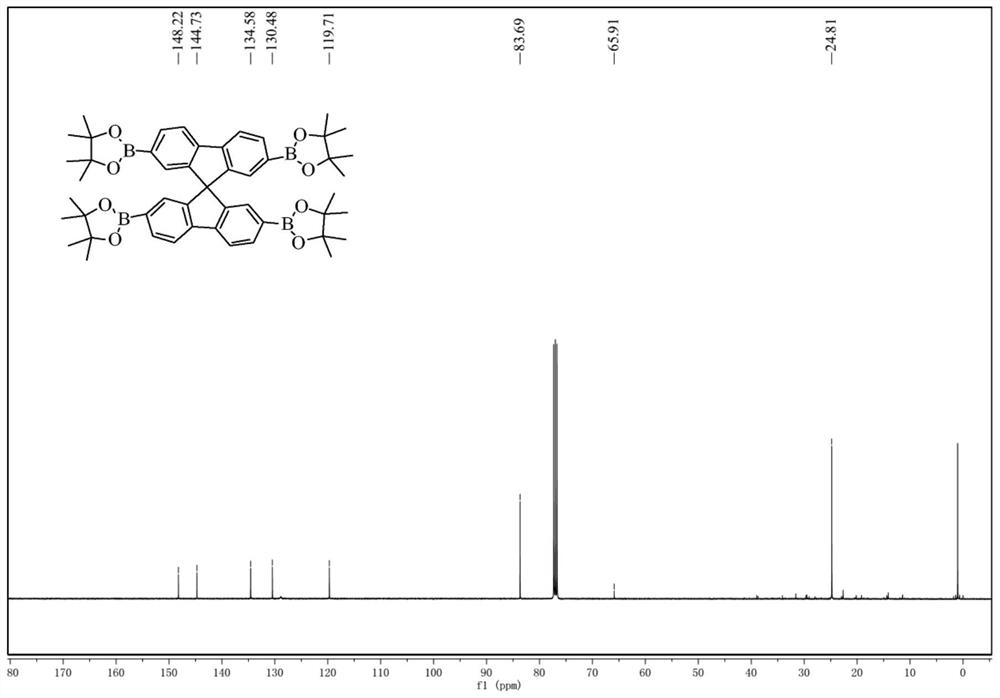
[0033] Under nitrogen, 2,2',7,7'-tetrabromospirofluorene (1.26 g, 2.0 mmol), pinacol diboronate (3.05 g, 12.0 mmol), potassium acetate (1.88 g, 18.0 mmol) were combined ) and 1,4-dioxane (30mL) were added to a 100mL reaction flask, followed by Pd (dppf 2 ) 2 (0.30 g, 0.4 mmol) catalyst, reacted at 100 °C overnight. The reaction was quenched with ice water, the organic phase was extracted with dichloromethane, washed two or three times with deionized water, dried over anhydrous magnesium sulfate, and filtered with suction. The crude product was concentrated in vacuo, and purified by column chromatography (eluent is DCM) to obtain 0.95 g of spirofluorene tetraborate as a white solid with a yield of 58%.
[0034] like figure 1 , 2 shown, 1 H NMR (400MHz, CDCl 3 ,δ): 7.85(s, 8H), 7.09(s, 4H), 1.25(s, 48H). 13 C NMR: (100MHz, CDCl 3 ,δ): 148.22, 144.73, 134.58, 130.48, 128.86, 119.71, 83.69, 65.91, 24.81.
example 2
[0035] Example 2: Preparation of pyrene tetraborate
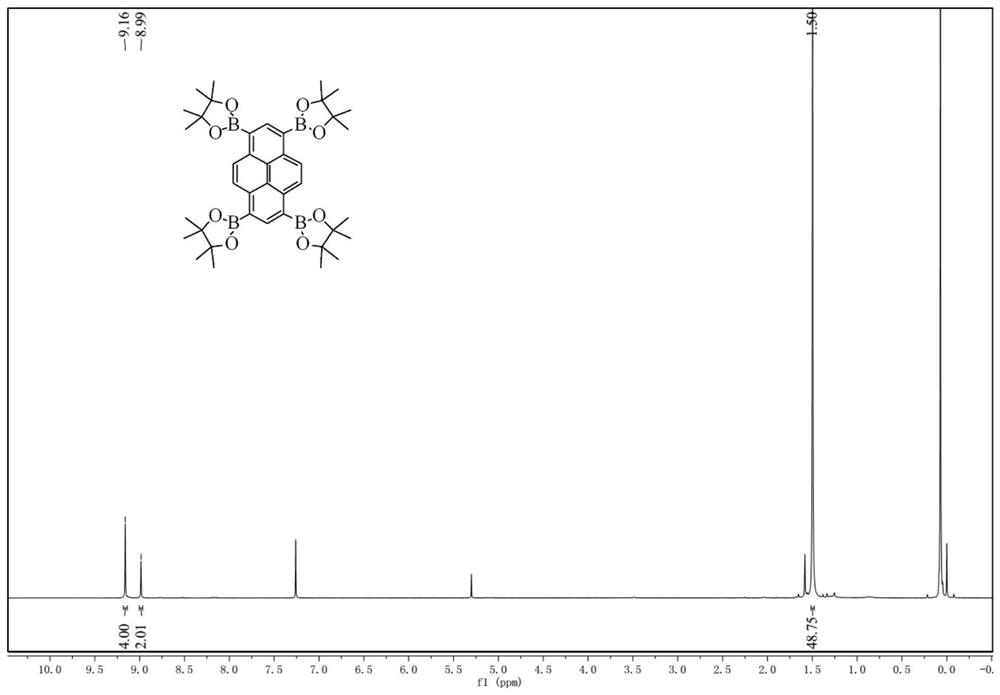
[0036] Under nitrogen, 1,3,6,8-tetrabromopyrene (1.20 g, 2.3 mmol), pinacol diboronate (3.50 g, 13.8 mmol), potassium acetate (1.98 g, 20.0 mmol) and 1 ,4-dioxane (30mL) was added to a 100mL reaction flask, followed by Pd (dppf 2 ) 2 (0.30 g, 0.4 mmol) catalyst, reacted at 100 °C overnight. The reaction was quenched with ice water, the organic phase was extracted with dichloromethane, washed two or three times with deionized water, dried over anhydrous magnesium sulfate, and filtered with suction. The crude product was concentrated in vacuo, and purified by column chromatography (eluent is DCM) to obtain pyrene tetraborate as a yellow solid, 0.76 g, in a yield of 47%.
[0037] like image 3 , 4 shown, 1 H NMR (400MHz, CDCl 3 ,δ): 9.16(s, 4H), 8.99(s, 2H), 1.50(s, 48H). 13 C NMR (100MHz, CDCl 3 ,δ): 141.32, 141.31, 137.97, 129.41, 123.98, 83.82, 25.09.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com