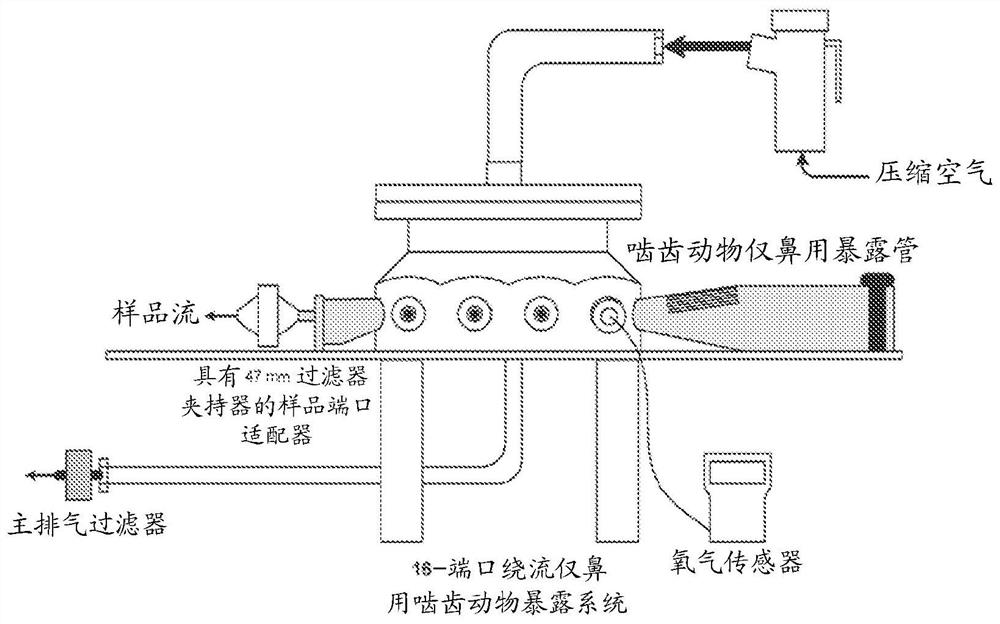
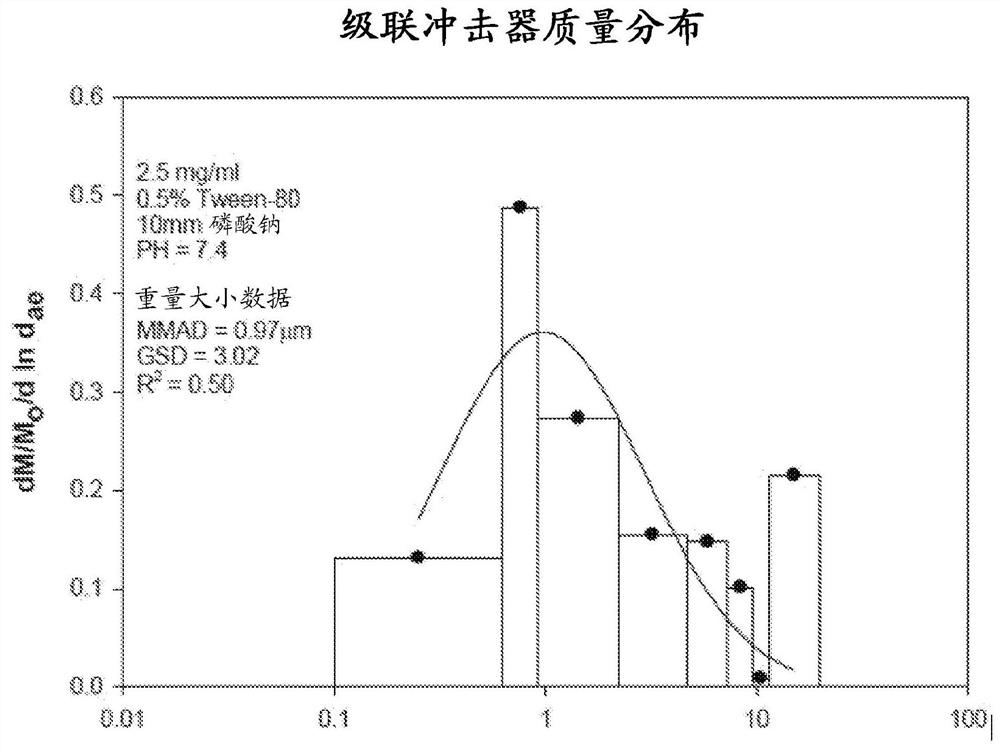
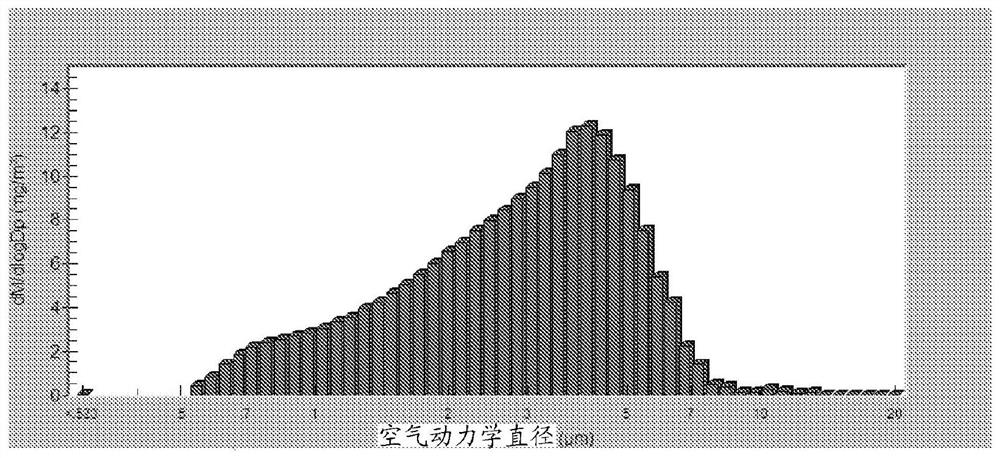
Bismuth-thiol compositions and methods of use
A composition and compound technology, applied in botany equipment and methods, active ingredients of heavy metal compounds, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0229] 1. A method for treating, managing a subject's cystic fibrosis (CF) symptoms and infection or reducing the severity of a subject's cystic fibrosis (CF) symptoms and infection, said method comprising adding to said The subject is administered a bismuth-thiol (BT) composition comprising at least one BT compound.
[0230] 2. The method of embodiment 1, wherein the subject has at least one pulmonary infection.
[0231] 3. The method of embodiment 2, wherein the subject has at least two pulmonary infections and the infections are concurrent or sequential.
[0232] 4. The method of embodiment 2 or 3, wherein the pulmonary infection is in one lung.
[0233] 5. The method of any one of embodiments 2-4, wherein the pulmonary infection is in both lungs.
[0234] 6. The method of any one of embodiments 2-5, wherein the pulmonary infection is in one or more of the three lobes of the right lung.
[0235] 7. The method of any one of embodiments 2-6, wherein the pulmonary infection...
Embodiment 1
[0453] Example 1: General Synthesis of Bis-EDT
[0454] Starting materials and reagents for the preparation of these compounds can be obtained from commercial suppliers such as Aldrich Chemical Co., Bachem, etc., or can be prepared by methods well known in the art. When necessary, the starting materials and intermediates and final products of the reactions can be isolated and purified using conventional techniques including, but not limited to, filtration, distillation, crystallization, chromatography, etc. Characterized in a conventional manner. Unless otherwise specified, the reactions described herein take place at atmospheric pressure at temperatures ranging from about -78°C to about 150°C.
[0455]
[0456] Particulate bismuth-1,2-ethanedithiol (Bis-EDT, soluble bismuth formulation) was prepared by adding an excess (11.4 L) of 5% HNO in a 15 L polypropylene carboy at room temperature by dropwise addition with stirring 3 Slowly add 0.331L (about 0.575 mole) Bi(NO 3 )...
Embodiment 2
[0459] Embodiment 2: Preparation of particulate bismuth-1-2-ethanedithiol (Bis-EDT)
[0460] Particulate bismuth-1,2-ethanedithiol (Bis-EDT) was prepared as follows: Water (25.5 L) and 70% nitric acid (1800 mL) were mixed together in a Nalgene reactor. Next, water (2300 mL) was added to the Erlenmeyer flask, followed by bismuth subnitrate (532 g), and the mixture was stirred. To the mixture was added 70% nitric acid (750 mL) to obtain a clear solution. This solution was transferred to the Nalgene reactor and the resulting mixture was stirred for 20 min. Next, 9.5 L of 95% EtOH was added to the reactor in three portions.
[0461] Separately, 98% 1,2-ethanedithiol (229 mL) was added to one bottle, followed by two 250 mL portions of EtOH with stirring. An additional 5 L of EtOH was added to the bottle with stirring. The 1,2-ethanedithiol solution was then added to the reactor with stirring over about 4 hours. After stirring for 18 hours, the solids were allowed to settle for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Volume mean diameter | aaaaa | aaaaa |
| Volume mean diameter | aaaaa | aaaaa |
| Volume mean diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com