Targeted delivery of therapeutic agents to human adipocytes
A fat cell and therapeutic agent technology, applied in the fields of behenic acid and acylglycine, can solve the problems of poor regeneration of liver cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
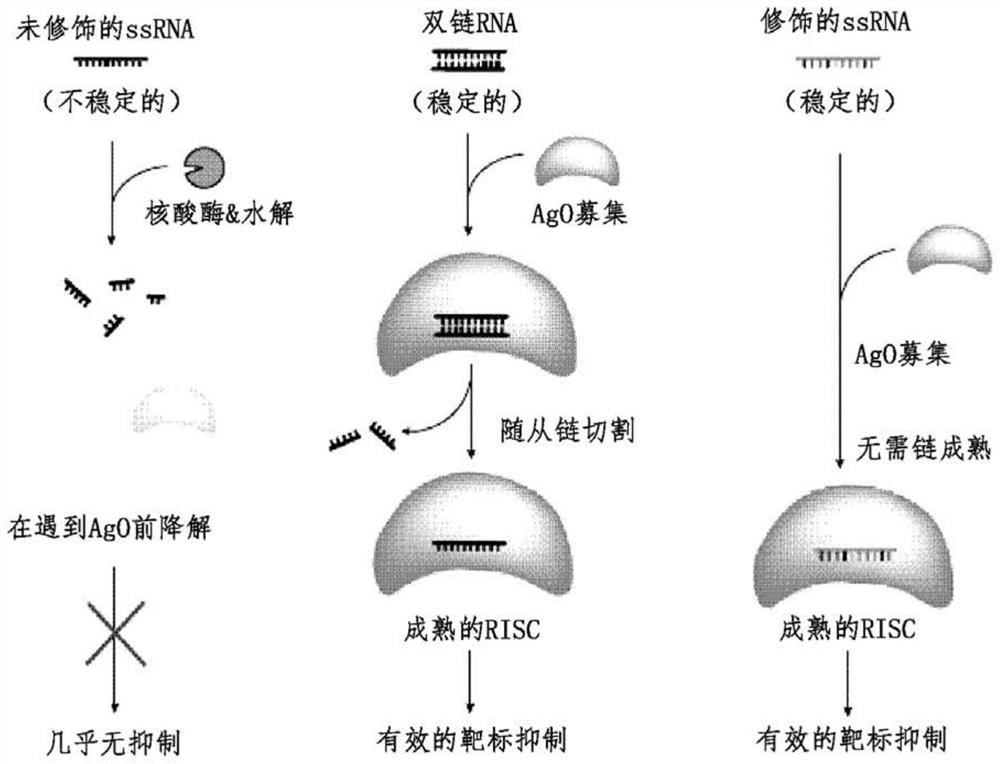
[0108] A. Example 1: Single-stranded miRNA (ss-miRNA) analogs with modified structure and length
[0109] Considering the critical role of the seed region (bases 2–8) and the interaction with the Ago2 protein required for the ss-miRNA to function, ss-miRNA analogs of different lengths were prepared including the following chemical modifications: 5' -(E)-vinylphosphonate protection (5'-VP), phosphorothioate (PS) backbone, 2'F, 2'-O-Me and 2'-O-MOE modifications, 5-methyl Modification of cytosine, introduction of a "pyrimidine box", and introduction of a "DNA gap" to make a "gapmer". like Figure 4-Figure 6 shown that either alone (“naked”) or in combination with a lipid tag (e.g., cholesterol or increased-length fatty acids) or a short peptide tag (e.g., hexarelin), the These molecules were tested in primary culture models of the ultimate target of anti-obesity drugs.
Embodiment 2
[0110] B. Example 2: Fatty Acid / miRNA Conjugates
[0111] An extensive "omic" analysis of mature human subcutaneous adipocytes and their exosomes was performed. The present inventors observed high-level expression of fatty acid translocase (FAT, also known as scavenger receptor B3 or CD36), an integral cell membrane transporter involved in the uptake of fatty acids into adipocytes [19, 22-25]. It is expected that combining ss-miRNA analogs with fatty acids naturally transported by FAT will facilitate the targeted delivery of such ss-miRNA analogs to mature adipocytes. This will be demonstrated by synthesizing and validating a series of miRNA analogs covalently linked to fatty acids to facilitate preferential targeting and translocation of adipocyte FAT. These miRNA analogs are referred to herein as AdipomiRs (miRNAs targeting adipocytes).
[0112] AdipomiR synthesis: Fatty acids have been used as chemical penetration enhancers (CPE) for various drugs, including oligonucleotide...
Embodiment 3
[0120] C. Example 3: Hexarelin / miRNA Conjugates
[0121] Recently, hexarelin (a chemically stable and potent growth hormone secretagogue His-D-2-Me-Trp-Ala-Trp-D-Phe-Lys-NH 2 , molecular formula: C 47 h 58 N 12 o 6 , molecular weight: 887) has beneficial effects on fat metabolism via the FAT / CD36 transporter [28, 29], causing fatty acid mobilization and activation of mitochondrial oxidative phosphorylation and thermogenesis. In MKR insulin-resistant mice, hexarelin treatment significantly improved glucose and insulin tolerance and decreased plasma and hepatic triglycerides. Furthermore, the cardioprotective effect of hexarelin is well documented [30].
[0122] Combining ss-miRNA analogs with hexarelin, which is naturally transported by FAT, is expected to facilitate the targeted delivery of such ss-miRNA analogs to mature adipocytes. Therefore, a series of miRNA analogs covalently linked to hexarelin to facilitate preferential targeting of adipocyte FAT was synthesized a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com