Modified polyaspartic acid ester resin and synthesis method thereof
A technology of aspartic acid ester and synthesis method, which is applied in the field of modified polyaspartic acid ester resin and its synthesis, and can solve the problems of increased processing difficulty, increased viscosity, low adhesion between paint and metal substrate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The second aspect of the present invention provides a kind of synthetic method of described modified polyaspartic acid ester resin, it comprises the following steps:
[0028] (1) Aliphatic diamine and diethyl maleate react at 65-90° C. to obtain polyaspartic acid ester resin;
[0029] (2) Add silicone-modified or unmodified epoxy resin and continue the reaction to obtain the product.
[0030] In one embodiment, the step (1) includes: reacting the aliphatic diamine and diethyl maleate at 65-90°C, and stopping the reaction when the amine conversion rate in the aliphatic diamine is 80-95mol%. Reaction to remove unreacted diethyl maleate to obtain polyaspartic acid ester resin containing unreacted aliphatic diamine.
[0031] Preferably, the mol ratio of the aliphatic diamine and diethyl maleate is 1:(2-3); more preferably, the mol ratio of the aliphatic diamine and diethyl maleate is 1 :2.
[0032] The applicant found in experiments that the reaction speed of aliphatic d...
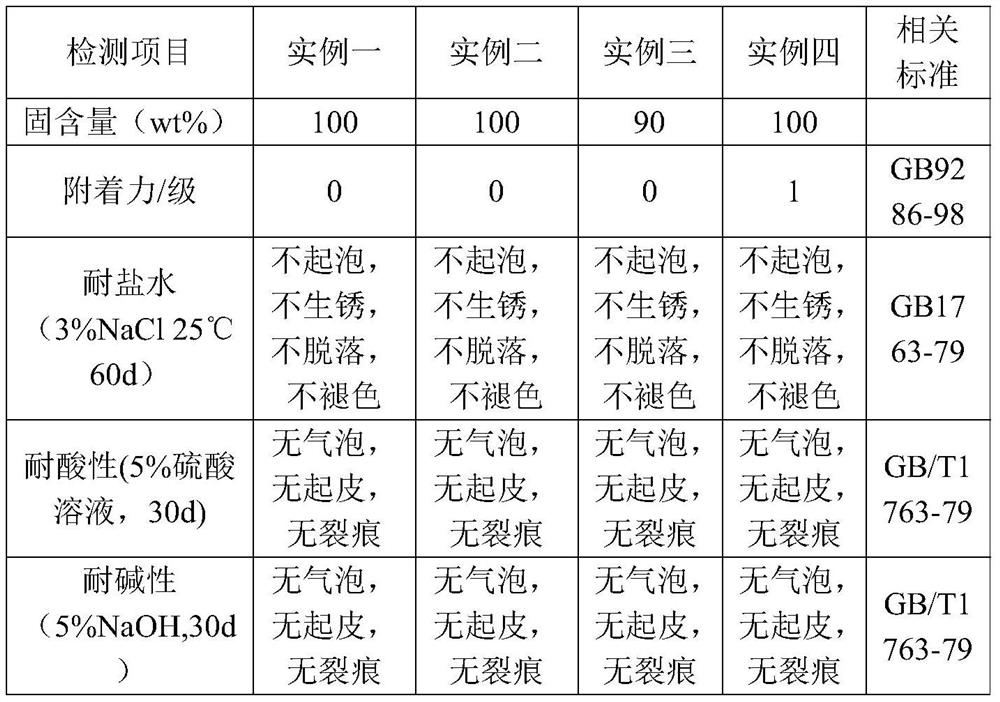
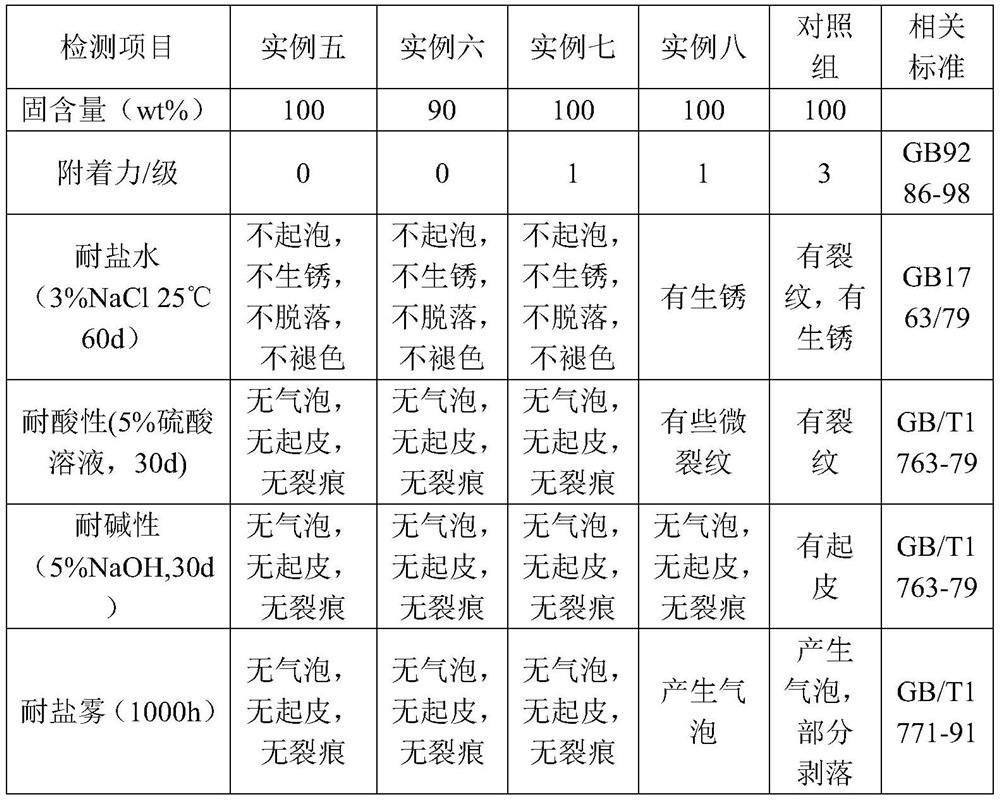
Embodiment 1
[0057] The synthetic method of polyaspartic acid ester resin in the embodiment of the present invention 1, specifically as follows:
[0058] (1) Add 52.5g (0.25mol) 4,4'-diaminocyclohexylmethane (HMDA) to a 500ml four-necked flask, add 86.1g (0.5mol) of diethyl maleate dropwise to HMDA, and pass Into N 2 , magnetically stirred, Michael addition reaction was carried out at 80°C, and the reaction was stopped when the conversion rate of 4,4'-diaminocyclohexylmethane reached 90 mol%, and the unreacted diethyl maleate was distilled off under reduced pressure to obtain the product X 1 ;
[0059] (2) Add 20.1g 0.5wt% silicone modified E51 resin, add the remaining 4,4'-diaminocyclohexylmethane from reaction (1) to the modified epoxy resin, and synthesize the target product Y 1 , and the yield was 98.8 wt%.
Embodiment 2
[0061] The synthetic method of polyaspartic acid ester resin in the embodiment of the present invention 2, specifically as follows:
[0062] (1) Add 52.5g (0.25mol) 4,4'-diaminocyclohexylmethane (HMDA) to a 500ml four-necked flask, add 86.1g (0.5mol) of diethyl maleate dropwise to HMDA, and pass Into N 2 , magnetically stirred, Michael addition reaction was carried out at 80°C, and the reaction was stopped when the conversion rate of 4,4'-diaminocyclohexylmethane reached 90 mol%, and the unreacted diethyl maleate was distilled off under reduced pressure to obtain the product X 2 .
[0063] (2) Add 20.4g of 2% silicone-modified E51 resin, add the remaining 4,4'-diaminocyclohexylmethane from reaction (1) to the modified epoxy resin, and synthesize the target product Y 2 , and the yield was 97.3 wt%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com