Application of naringenin and naringenin composition in preparation of medicine for treating or preventing toxoplasmosis
A technology of toxoplasmosis and naringenin, which is applied in the preparation of drugs for the treatment or prevention of toxoplasmosis, in the field of naringenin, achieves the effects of low toxicity, convenient treatment or prevention, and prevention of toxoplasmosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
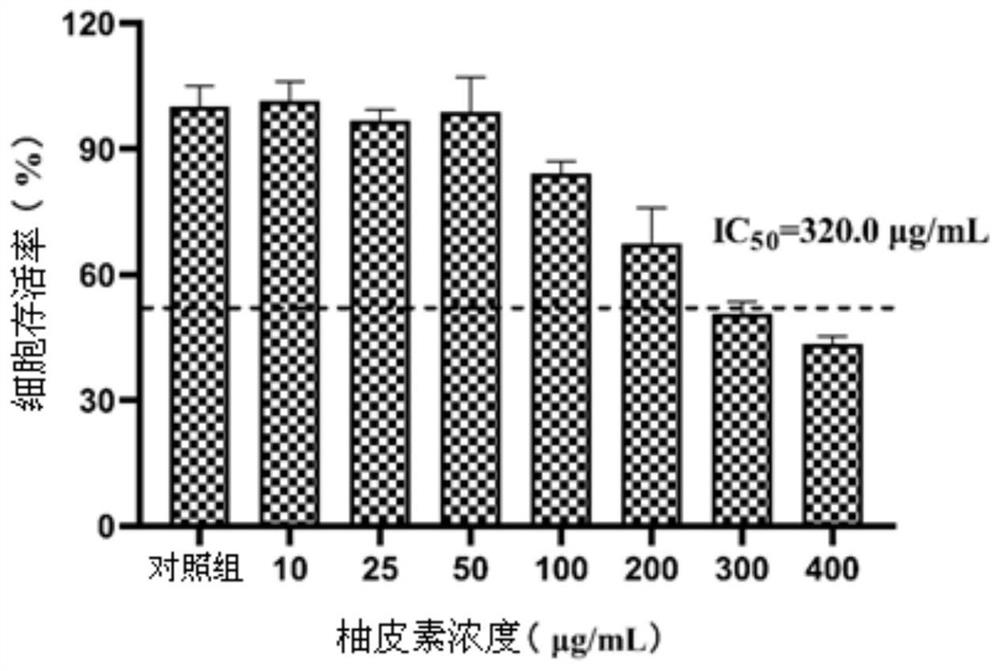
[0023] Example 1 Toxicity of naringenin to Vero cells
[0024] 1. Experimental process
[0025] The CCK-8 method was used to determine the toxicity of naringenin to Vero cells.
[0026] Adjust the concentration of Vero cells to 1 x 10 5 cells / mL, inoculate them in 96-well plates, and after culturing for 12 hours, add different concentrations of naringenin solutions respectively, and use 0.25% DMSO as the control group, and use pure medium as the blank group. After culturing for 24 hours, CCK-8 reagent was added, and after acting for 1 hour, the optical density OD value of each well was measured in a 450 nm microplate reader. Calculate the survival rate of Vero cells according to the following formula, and draw the curve and calculate the IC 50 value.
[0027] Cell viability (%) = (OD 试验组 -OD 空白组 ) / (OD 对照组 -OD 空白组 )×100%
[0028] 2. Experimental results
[0029] The above-mentioned naringenin solutions of different concentrations are calculated on the survival rate of...
Embodiment 2
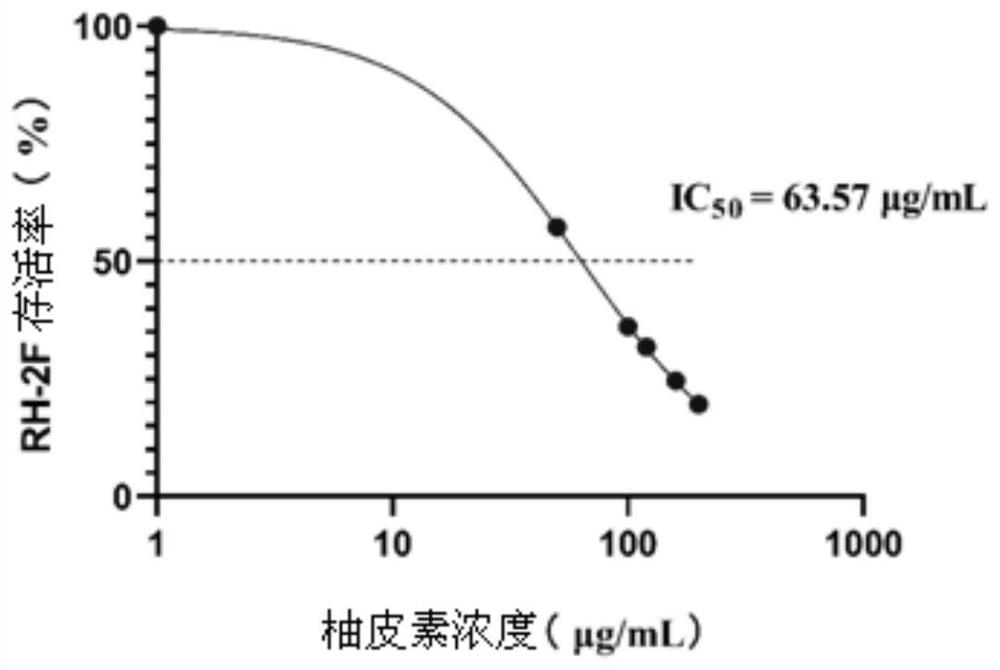
[0033] The inhibitory effect of embodiment two naringenin to Toxoplasma gondii
[0034] 1. Experimental process
[0035] Toxoplasma gondii RH-2F strain expressing β-galactosidase was used for growth inhibition assay.
[0036] Harvest fresh and vigorous Toxoplasma gondii RH-2F tachyzoites from Vero cells, inoculate them in 96-well plates, count on a hemocytometer, and adjust the concentration of Toxoplasma gondii RH-2F tachyzoites to 1×10 5 individual / mL. Different concentrations of naringenin solutions were prepared and added to 96-well plates respectively, with 0.25% DMSO as the control group and pure medium as the blank group. After 12 hours of incubation, add As for the detection reagent, after acting for 30 minutes, detect the luminescence value in a photometer. Calculate the survival rate of Toxoplasma gondii RH-2F tachyzoites, draw the curve and calculate the IC 50 value.
[0037] 2. Experimental results
[0038] The above-mentioned naringenin solutions of differ...
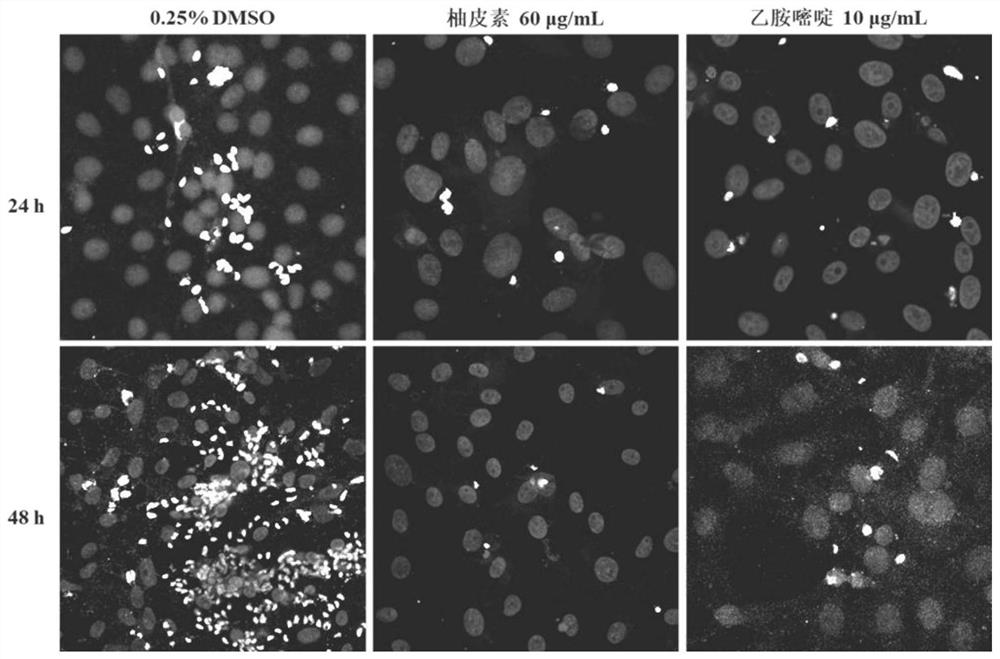
Embodiment 3
[0044] The anti-proliferation effect of embodiment three naringenin on intracellular Toxoplasma gondii type I strain (RH)
[0045] 1. Experimental process
[0046] Will 1×10 5 RH tachyzoites / mL of the Toxoplasma gondii type I strain were inoculated in a monolayer of Vero cells in a 12-well plate. After invading for 4 hours, a naringenin solution with a concentration of 60 μg / mL was added and mixed with 0.25% DMSO As the negative control group, 10 μg / mL pyrimethamine was used as the positive control group for 24 h and 48 h, then fixed with 4% paraformaldehyde, antigen retrieval, blocked with 10% goat serum, permeabilized with 0.2% Triton X-100, Toxoplasma was labeled with rabbit anti-toxoplasma polyclonal antibody, the secondary antibody was goat anti-rabbit IgG H&L, and the nuclei were stained with DAPI. After staining and mounting, the slides were observed and photographed under a confocal microscope.
[0047] 2. Experimental results
[0048] attached image 3 It is the pi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com