N-terminal extension type PTEN subtype PTEN zeta protein as well as coding gene and application thereof
An N-terminal, protein technology, applied to the N-terminal extended PTEN isoform PTENζ protein and its encoding gene and application fields, can solve the problems of low translation initiation ability and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Translation of PTENζ protein starts at ATT 948 identification method
[0059] (1) Using plasmid expression and mutation analysis to show that the translation of PTENζ starts from ATT 948
[0060] According to the identification of some PTEN subtype proteins such as PTENα, PTENβ and other new subtype proteins of PTEN in the previous research work, in the process of research on PTEN and subtype proteins, a slightly higher molecular weight position (55kDa) than the traditional PTEN protein was found. Bands of unknown proteins (see figure 1 , see [1] PTENα, aPTEN Isoform translated through alternative initiation, regulates mitochondrial function and energy metabolism, Hui Liang, Shiming He, Jingyi Yang, et al. Cell Metabolism, 19, 836-848, 2014, May 6. [2] PTENβ is an alternatively translate isoform of PTEN that regulates rDNA transcription, Hui Liang, Xi Chen, Qi Yin, Danhui Ruan, et al. Nature Communications, 2017.).
[0061]Subsequently, different tumor cell lines we...
Embodiment 2
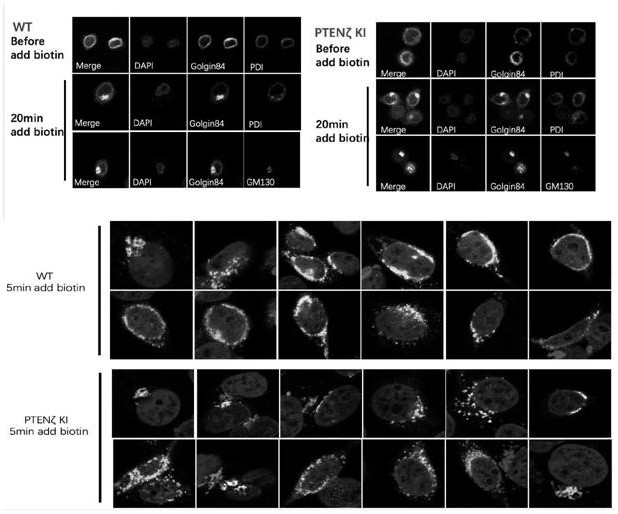
[0074] Physiological functions of PTENζ
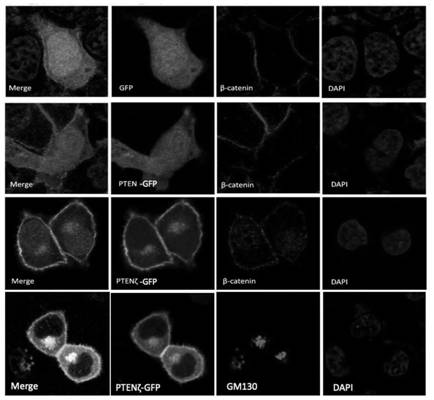
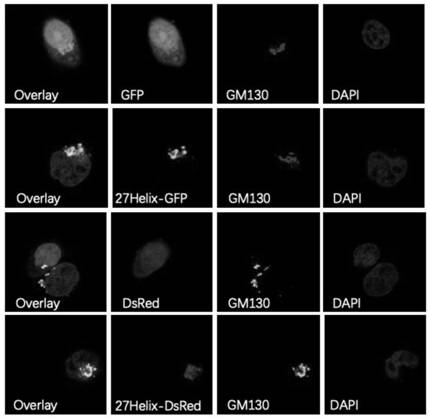
[0075] (1) PTENζ is located in the Golgi apparatus, and its N-terminal 28 amino acid protein sequence is a Golgi apparatus positioning signal
[0076] The function of a protein is closely related to its localization. In order to analyze the function and molecular mechanism of PTENζ, reveal whether PTENζ and traditional PTEN proteins have the same subcellular localization. The nucleotide sequences of PTENζ and traditional PTEN protein were amplified from the cDNA of Hela cells respectively, and the kozak sequence (GCCACCATG) was added upstream of the start codon to enhance expression, and then ligated into GFP carrying the C-terminal by homologous recombination. Label within the pEGFP-N1 vector. At the same time, in order to enhance the expression of this subtype protein, the variable translation initiation codon (ATT 948 ) was mutated to ATG and the start codon ATG of the traditional PTEN protein in the plasmid was mutated to CTC, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com