Preparation method of favipiravir intermediate 2-aminomalonamide
A technology of aminomalonamide and favipiravir, which is applied in the preparation of carboxylic acid amides, organic compounds, cyanide reaction, etc., can solve poisoning inactivation, complicated production process, palladium carbon can not play a role Catalytic hydrogenation and other issues to achieve the effect of not easy to deactivate, reduce production cost, and good tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Prepare 2-amino malonamide as follows:
[0033] (1) Take 150g of diethyl 2-nitrosomalonate, add 400g of methanol, stir and dissolve, add 33g of Raney nickel, replace with hydrogen three times, control the reaction pressure at 0.5-1MPa, and heat up to 40-45°C for reaction;
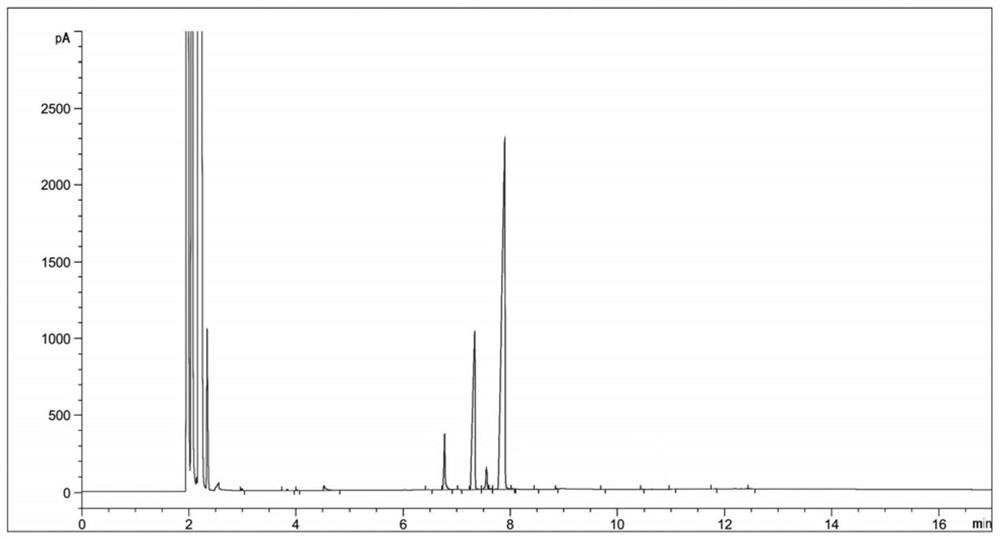
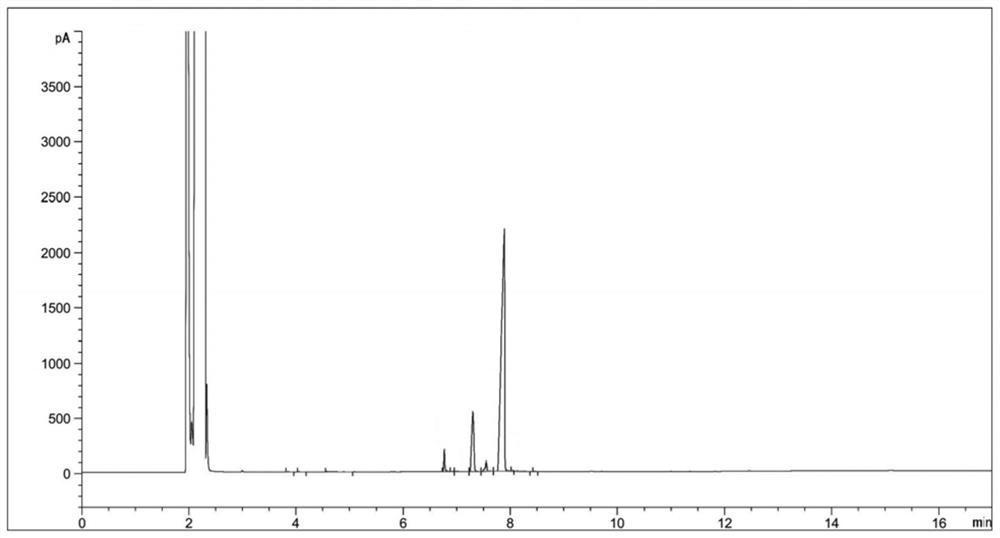
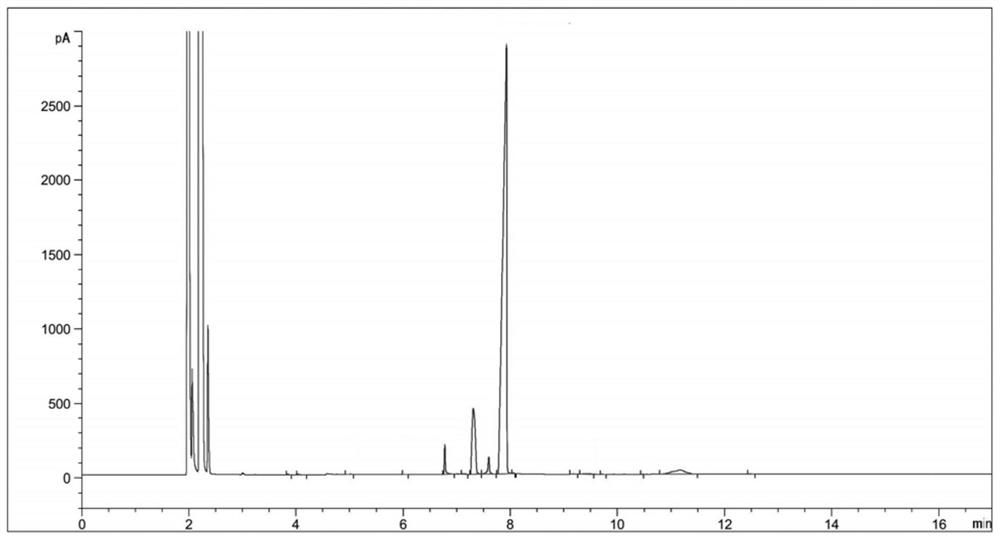
[0034] (2) Sampling and detection after 6 hours of reaction, the reaction of diethyl 2-nitrosomalonate is complete (there are three main peaks in the reaction solution at this time, respectively RT=6.77, RT=7.33, RT=7.89, the corresponding products dimethyl 2-aminomalonate, ethyl 2-aminomalonate and compound Ⅱ. The reason for the three main peaks is that the reaction solvent is methanol, and the transesterification between methanol and compound Ⅱ generates a single Methyl ester and bismethyl ester products will be transformed into 2-aminomalonamide during the subsequent aminolysis reaction), the temperature is lowered to 20-30°C, and the hydrogenation reaction solution is obtained by filtering;
[0...
Embodiment 2
[0042] Prepare 2-amino malonamide as follows:
[0043] (1) Take 150g of diethyl 2-nitrosomalonate, add 400g of methanol, stir and dissolve, add 33g of Raney nickel, replace with hydrogen three times, control the reaction pressure at 0.5-1MPa, and heat up to 40-45°C for reaction;
[0044] (2) Sampling and testing after 4 hours of reaction, the reaction of diethyl 2-nitrosomalonate was complete, the temperature was lowered to 20-30°C, and the hydrogenation reaction liquid was obtained by filtering;
[0045] (3) Concentrate the hydrogenation reaction solution to obtain 124 g of concentrated solution; sampling is carried out for HPLC detection, and the detection results are shown in Table 2;
[0046] (4) Control the temperature of the concentrated solution to be 10-20°C, add 550ml of saturated ammonia methanol solution dropwise to the concentrated solution, and react at a temperature of 15-20°C; after reacting for 1h, take a sample to detect the content of ammonia in the reaction ...
Embodiment 3
[0052] Prepare 2-amino malonamide as follows:
[0053] (1) Take 150g of diethyl 2-nitrosomalonate, add 400g of methanol, stir and dissolve, add 33g of Raney nickel, replace with hydrogen three times, control the reaction pressure at 0.5-1MPa, and heat up to 40-45°C for reaction;
[0054] (2) Sampling and testing after 4 hours of reaction, the reaction of diethyl 2-nitrosomalonate was complete, the temperature was lowered to 20-30°C, and the hydrogenation reaction liquid was obtained by filtering;
[0055] (3) Concentrate the hydrogenation reaction solution to obtain 130 g of concentrated solution; sampling is carried out for HPLC detection, and the detection results are shown in Table 3;
[0056](4) Control the temperature of the concentrated solution to be 10-20°C, add 550ml of saturated ammonia methanol solution dropwise to the concentrated solution, and react at a temperature of 15-20°C; after reacting for 1h, take a sample to detect the content of ammonia in the reaction s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com