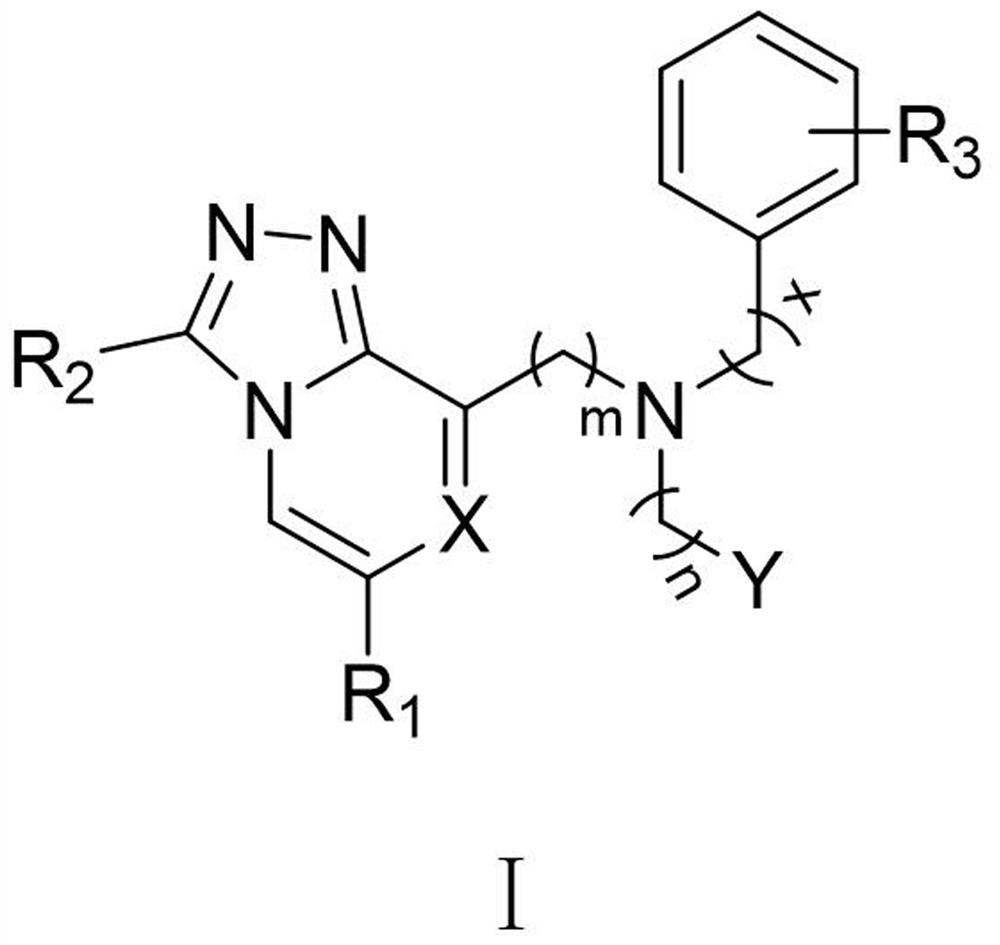
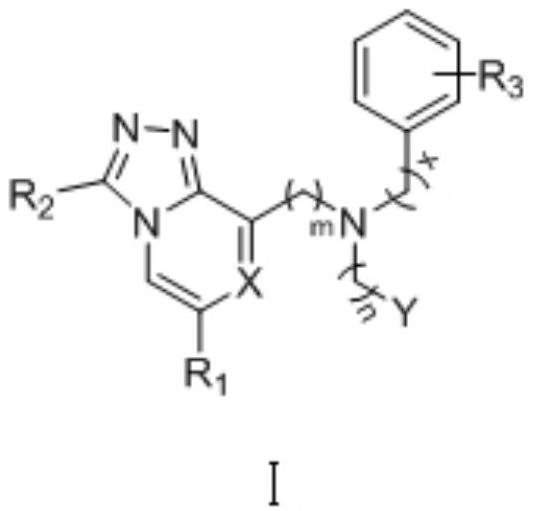
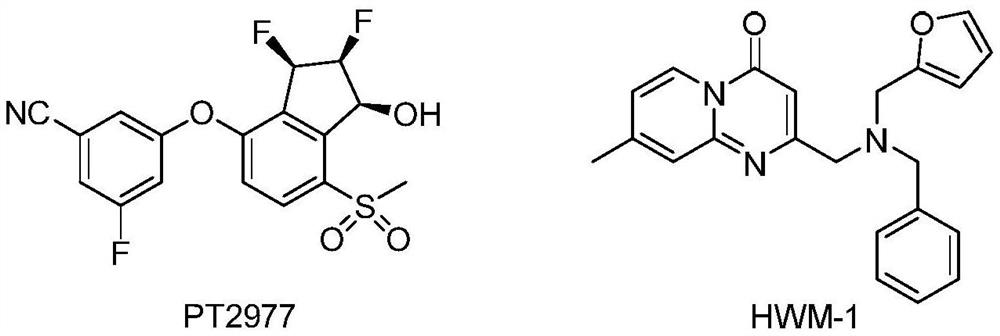
Preparation of HIF-2alpha small-molecule inhibitor and application thereof
A small molecule inhibitor, HIF-2 technology, applied in the field of medicine to achieve the effects of reduced toxicity, broad anti-cancer spectrum and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1 1-([1,2,4]Triazolo[4,3-a]pyrazin-8-yl)-N-benzyl-N-(furan-2-ylmethyl)methanamine ( Preparation of A1).
[0100] Step 1. Dissolve 3-bromo-2-chloropyrimidine (10g, 0.052mol) in toluene (200ml), cool down to -65°C, add DMF (dimethylformamide) (6.2ml, 0.08mol) dropwise , After dropping, n-butyllithium (1.57M solution in n-hexane; 51 mL, 0.08 mol) was added dropwise. After stirring for 20 minutes, the reaction was terminated with 1N hydrochloric acid, then the reaction solution was extracted with ethyl acetate, several layers were washed with water, dried over anhydrous magnesium sulfate, filtered, and concentrated to dryness to obtain the product (intermediate a) without further purification. in the next step.
[0101] Step 2. Sodium borohydride (2 g, 0.052 mol) was slowly added in batches to the solution of intermediate a dissolved in ethanol (30 mL) at room temperature, and stirred for 30 minutes after the addition. After quenching the reaction with 36% glacia...
Embodiment 2
[0107] Example 2 1-([1,2,4]Triazolo[4,3-a]pyrazin-8-yl)-N-(4-fluorobenzyl)-N-(furan-2-ylmethane yl) methylamine (A2).
[0108] The preparation method is the same as that of Example 1, ESI-MS m / z: 337.36; 1 H-NMR (400MHz, DMSO-d 6 )δ9.13(s,1H),8.36(d,J=2.7Hz,1H),7.68(d,J=2.7Hz,1H),7.44(dd,J=0.32Hz,2H),7.36(d, J=4.3Hz, 1H), 7.07(t, 2H), 6.52(d, J=0.88Hz, 1H), 6.30(t, J=0.88Hz, J=4.3Hz, 1H), 3.99(s, 2H) , 3.82(s, 2H), 3.54(s, 2H).
Embodiment 3
[0109] Example 3 1-([1,2,4]Triazolo[4,3-a]pyrazin-8-yl)-N-(3-bromophenyl)-N-(furan-2-ylmethane yl) methylamine (A3).
[0110] The preparation method is the same as that of Example 1, ESI-MS m / z: 398.26.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com