Electrolyte for lithium ion battery
A lithium-ion battery and electrolyte technology, applied in the field of lithium-ion batteries, can solve the problems of matching electron conduction rates, large ion transfer impedance, preventing high-power lithium-ion batteries, etc., and achieves low electrode interface impedance and high ion conduction capability, the effect of high power performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Electrolyte composition: solvent, electrolyte salt and additives.
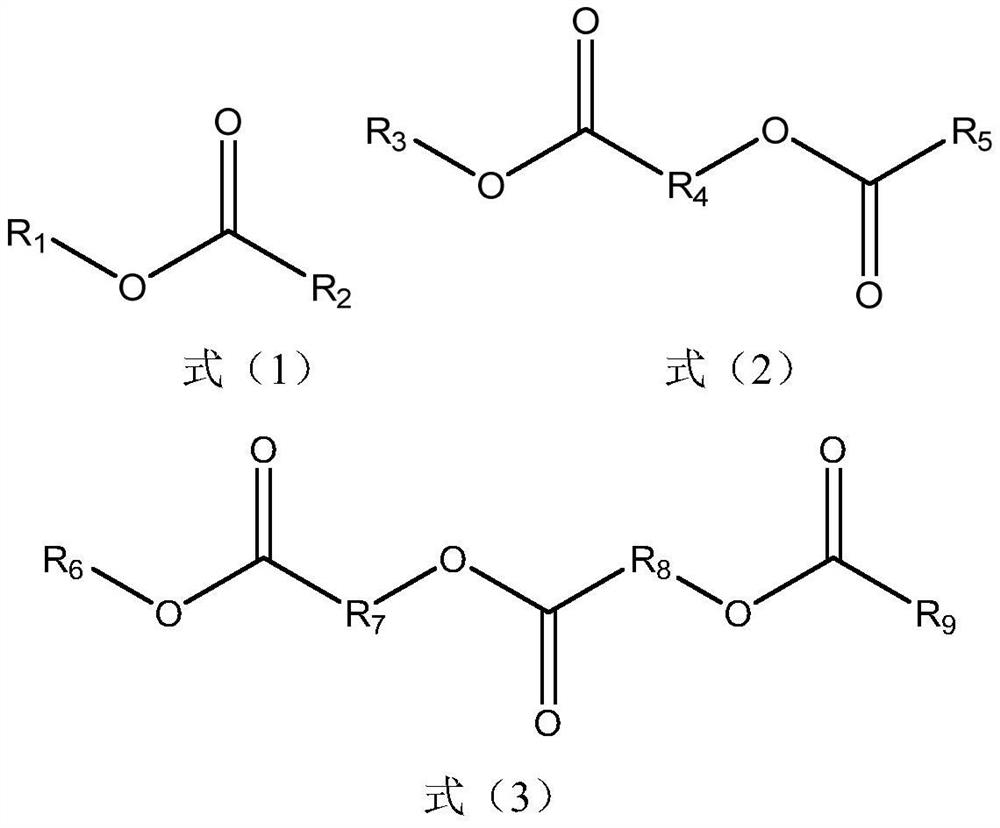
[0031] Solvent: ethylene carbonate, solvent (solvent structure 1) (volume ratio 1:1).
[0032] The electrolyte salt is lithium hexafluorophosphate, the concentration is 1mol / L,
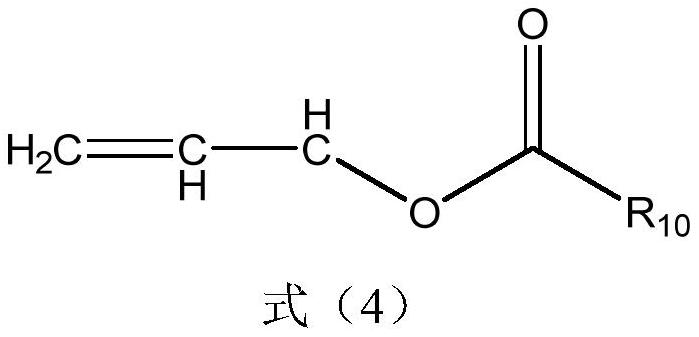
[0033] The additive is additive structure 1 with a concentration of 2%.
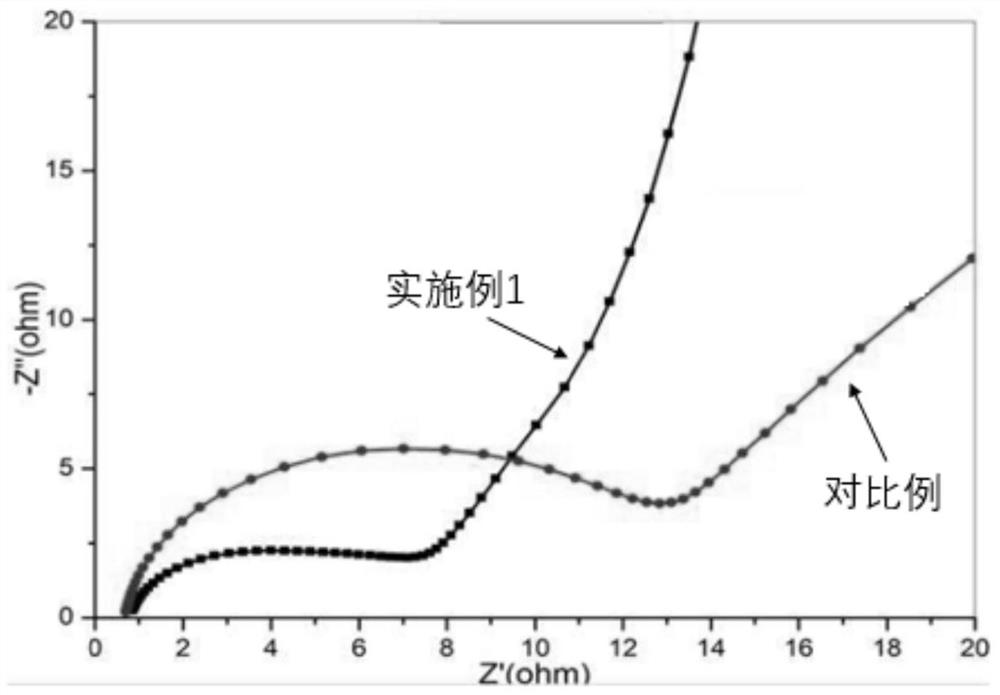
[0034] After mixing the above materials evenly, test the conductivity of the electrolyte. The positive electrode material used to test the performance of the electrolyte is lithium cobalt oxide, and the load is 4mg / cm 2 , the negative electrode material is graphite, and the load is 1.5mg / cm2. The separator is a polypropylene separator that composes a 2016 button cell. The charge and discharge currents are 10A, respectively, and the cut-off voltage is 3.0V-4.2V. The energy densities of the cells based on the active materials are reported in Table 2. And test the AC impedance of the battery, record in figure 1
Embodiment 2
[0038] Electrolyte composition. Solvent: ethylene carbonate, solvent (structure 2) (volume ratio 1:1.5). The electrolyte salt is lithium hexafluorophosphate with a concentration of 1.2 mol / L, and the additive is additive structure 2 with a concentration of 2%. After mixing the above materials evenly, test the conductivity of the electrolyte. The positive electrode material used to test the performance of the electrolyte is lithium cobalt oxide, and the load is 4mg / cm 2 , the negative electrode material is graphite, and the load is 1.5mg / cm2. The separator is a polypropylene separator that composes a 2016 button cell. The charge and discharge currents are 10A, respectively, and the cut-off voltage is 3.0V-4.2V. The energy densities of the cells based on the active materials are reported in Table 2.
Embodiment 3
[0040] Electrolyte composition. Solvent: ethylene carbonate, solvent (structure 3) (volume ratio 1:1). The electrolyte salt is lithium hexafluorophosphate with a concentration of 1.1 mol / L, and the additive is additive structure 3 with a concentration of 2%. After mixing the above materials evenly, test the conductivity of the electrolyte. The positive electrode material used to test the performance of the electrolyte is lithium cobalt oxide, and the load is 4mg / cm 2 , the negative electrode material is graphite, and the load is 1.5mg / cm2. The separator is a polypropylene separator that composes a 2016 button cell. The charge and discharge currents are 10A, respectively, and the cut-off voltage is 3.0V-4.2V. The energy densities of the cells based on the active materials are reported in Table 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com