Ptprs and proteoglycans in rheumatoid arthritis
A technology of immunoglobulins and analogs, applied in the direction of anti-animal/human immunoglobulins, immunoglobulins, peptides/protein components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0187] Example 1. Materials and methods
[0188] polypeptide
[0189] The following peptides were used in the experiments:
[0190] "In use" (SEQ ID NO: 16):
[0191] EEPPRFIREPKDQIGVSGGVASFVCQATGDPKPRVTWNKKGKKVNSQRFETIDFDESSGAVLRIQPLRTPRDENVYECVAQNSVGEITIHAKLTVLREDQLPPGFPNIDMGPQLKVVERTRTATMLCAASGNPDPEITWFKDFLPVDPSASNGRIKQLRSGALQIESSEETDQGKYECVATNSAGVRYSSPANLYVRTSGGGSLVPRGSEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKTISKAKGQPREPQVYTLPPSRDELTKNQVSLTCLVKGFYPSDIAVEWESNGQPENNYKTTPPVLDSDGSFFLYSKLTVDKSRWQQGNVFSCSVMHEALHNHYTQKSLSLSPGK
[0192] "Construct 1" (SEQ ID NO: 17):
[0193]ETGEEPPRFIREPKDQIGVSGGVASFVCQATGDPKPRVTWNKKGKKVNSQRFETIDFDESSGAVLRIQPLRTPRDENVYECVAQNSVGEITIHAKLTVLREDQLPPGFPNIDMGPQLKVVERTRTATMLCAASGNPDPEITWFKDFLPVDPSASNGRIKQLRSGALQIESSEETDQGKYECVATNSAGVRYSSPANLYVRTSGGGGSGGGGSEPKSCDKTHTCPPCPAPELLGGPSVFLFPPKPKDTLMISRTPEVTCVVVDVSHEDPEVKFNWYVDGVEVHNAKTKPREEQYNSTYRVVSVLTVLHQDWLNGKEYKCKVSNKALPAPIEKT...
Embodiment 2
[0257] Combination therapy targeting synoviocytes for rheumatoid arthritis
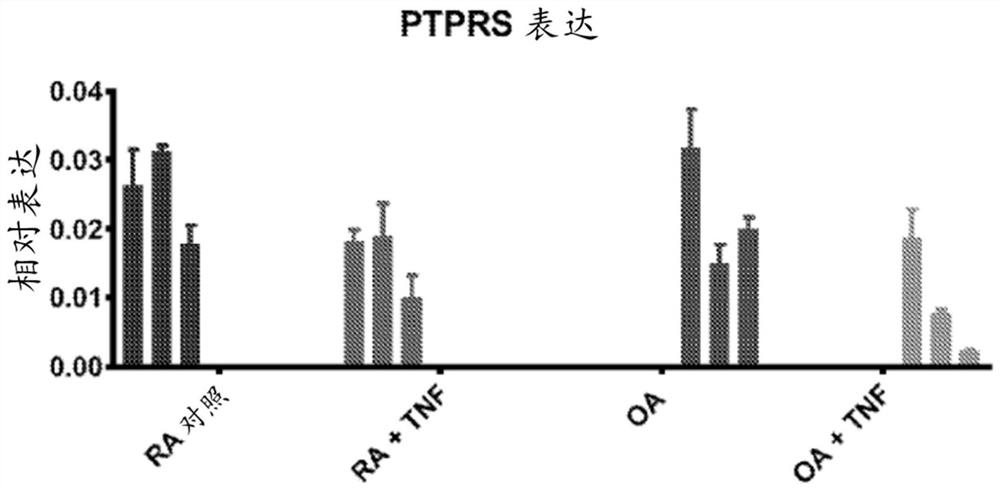
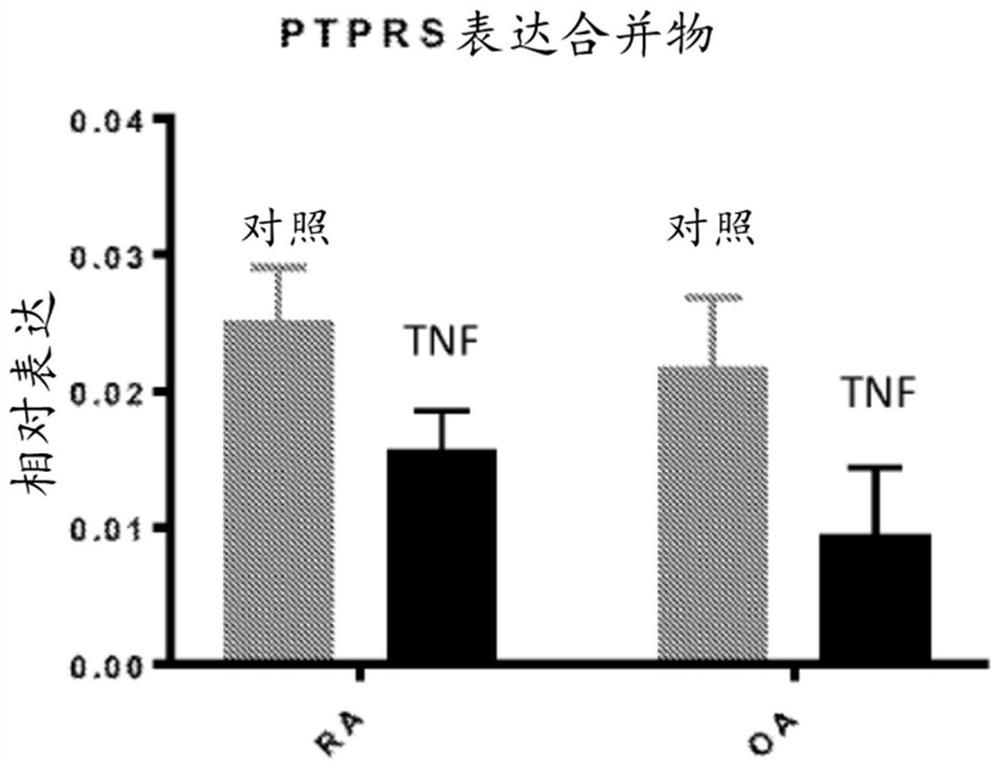
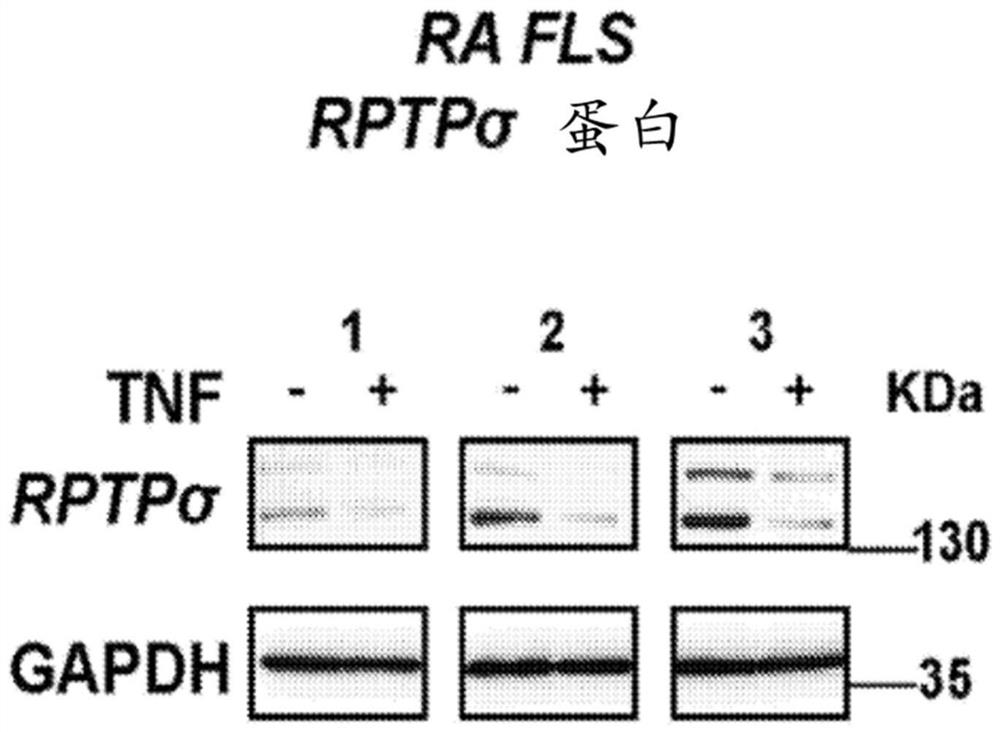
[0258] The effect of TNF on the expression of PTPRS (encoding RPTPσ) was investigated in rheumatoid arthritis (RA) and osteoarthritis (OA) fibroblast-like synoviocytes (FLS). FLS was used between passage 4 and 10, and cells were synchronized in 0.1% FBS (serum-starved medium) for 24-48h, then stimulated with 50ng / ml TNF for 24h. RNA was extracted using RNeasy kit (Qiagen). Use for qRT-PCR cDNA was synthesized by III First-Strand Synthesis SuperMix (Life Technologies). With primer assay and from SABiosciences / Qiagen Green qPCR Mastermix, qPCR was performed on the Bio-Rad CFX384 Real-Time PCR Detection System. The manufacturer guarantees that the primer assay efficiency is greater than 90%. Each reaction was measured in triplicate using the technique and the data were normalized to the expression level of the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Results are present...
Embodiment 3
[0275] Example 3. Synergistic reversal of arthritis by synoviocyte-targeted therapy and TNF immunomodulation
[0276] By treating mice with severe arthritis (clinical score of at least 8) with doses of Ig1&2 (0.1 mg) and murine etanercept (2 mg / kg) that were not effective in reversing severe arthritis as monotherapy, suboptimal Therapeutic synergy of therapeutic doses of Fc-Ig1&2 and murine etanercept in CIA. CIA was induced and scored as described above. Fc-Ig1 & 2 and murine etanercept were obtained and injected as described above. For histological scoring of mouse arthritic joints, whole hind paws were fixed in 10% formalin, decalcified, trimmed and embedded. Sections were prepared from tissue blocks and stained with hematoxylin and eosin, safranin-O or toluidine blue (HistoTox). Histopathological scoring was performed as described. Briefly, inflammation in the joints of arthritic mice was scored 0-4 based on hematoxylin-eosin staining according to the following criteri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com