A kind of peganumine A alkaloid structure simplification and its application
A technology of simplified substances and alkaloids, applied in the field of medicine, can solve the problems of further improvement of anti-tumor activity, limited sources of alkaloids, and high difficulty of total synthesis, and achieve simple chemical synthesis routes, easy synthesis methods, and excellent druggability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
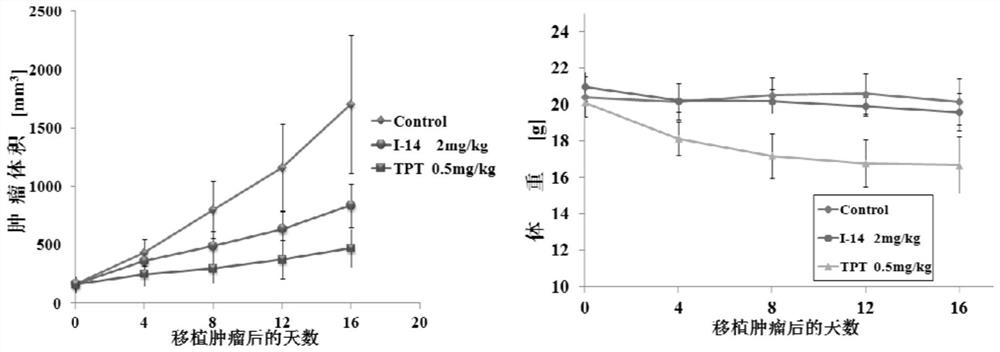
Examples
Embodiment 1
[0038] Synthesis of compound I-1
[0039]
[0040] The first step, the preparation of intermediate 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indole-1-carboxylic acid (compound 3)
[0041] According to the method reported in the literature (Organic Letters, 2014, 16, 4194-4197), in 50 ml of water, add tryptamine, compound 1 (1.0 g, 6.24 mM) and glyoxylic acid, compound 2 (0.47 g, 6.3 mM ), stirring and reacting at room temperature for 2 hours, a white precipitate was obtained, which was filtered by suction and dried in vacuo to obtain compound 3, 1.05 g of a white solid, with a yield of 77.8%.
[0042] The second step, the preparation of intermediate 6,7-dimethoxy-3,4-dihydroisoquinoline (compound 5)
[0043]
[0044] In 2 ml DMSO, add 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline, compound 4 (0.1 g, 0.52 mM) and 2-iodobenzoic acid (IBX, 0.15 g, 0.52 mM), react in DMSO at room temperature for 1 hour. After the reaction was complete, add saturated NaCl to wash with water, extra...
Embodiment 2
[0049] It was prepared according to the method steps of Example 1, the raw material of the first step was changed to compound 6, and the second and third steps were prepared according to the method of Example 1.
[0050]
[0051] Compound I-2 was obtained as a white solid 28 mg, with a yield of 27%.
Embodiment 3
[0053] It was prepared according to the method steps of Example 1, the raw material of the first step was changed to compound 8, and the second and third steps were prepared according to the method of Example 1.
[0054]
[0055] Compound I-3 was obtained as a white solid 45 mg with a yield of 45%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com