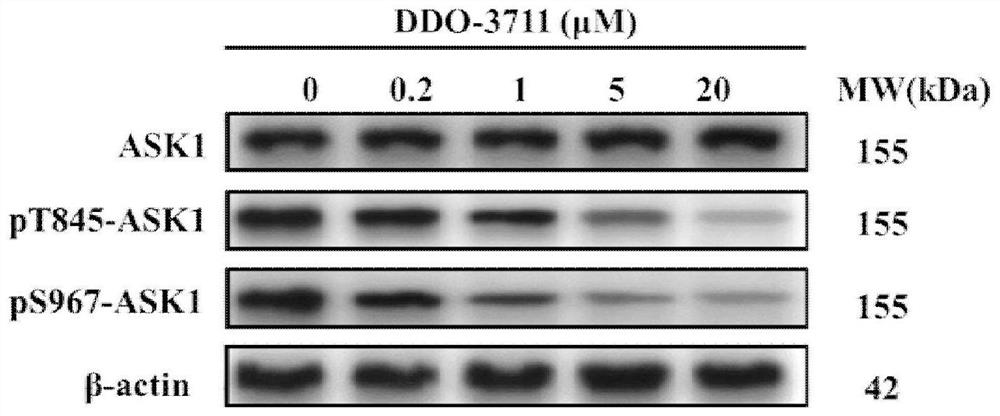
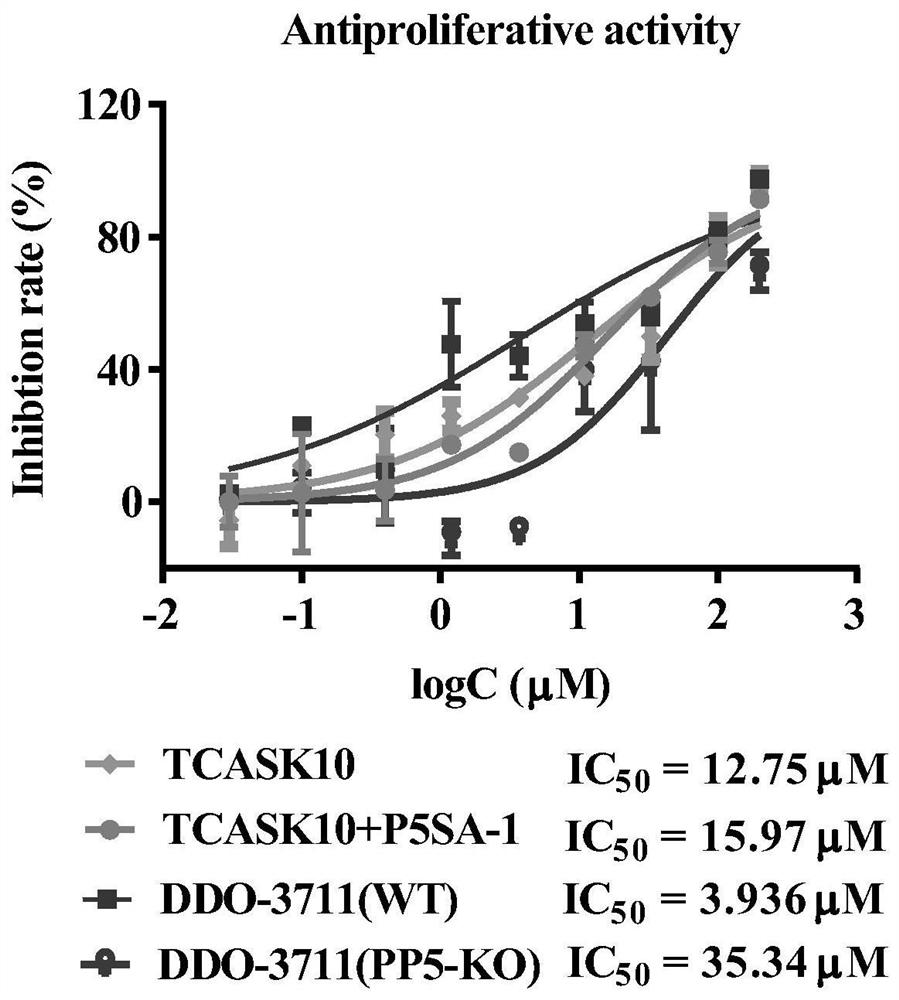
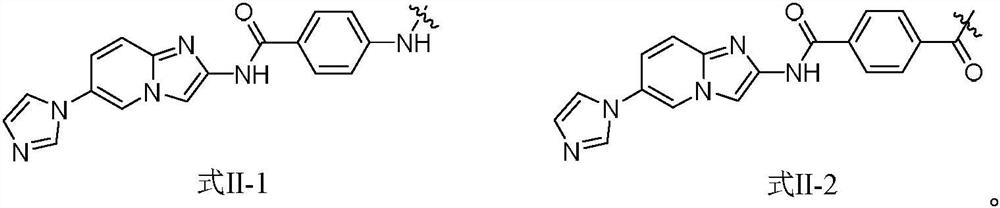
Protein phosphatase 5-based phosphatase recruitment chimera (PHORCs) compound as well as preparation method and medical application thereof
A protein phosphatase and compound technology, applied in the fields of drug combination, organic chemistry, anti-tumor drugs, etc., can solve the problems of accelerated disease process, abnormally active function, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation of N-(6-(1H-imidazol-1-yl)imidazo[1,2-a]pyridin-2-yl)-4-palmitoylaminobenzamide (DDO-3701).
[0038] synthetic route:
[0039]
[0040] Compound 1 (50 mg, 0.16 mmol) was dissolved in 3 ml N, N-dimethylformamide, palmitic anhydride (194 mg, 0.39 mmol), N, N-diisopropylethylamine (61 mg, 0.46 mmol) were added, Under the protection of nitrogen, react at 70°C for 5 hours; filter with suction and concentrate the filtrate; add 5ml of ethyl acetate for slurry, filter with suction, rinse with 2ml of ethyl acetate; dry the filter cake at 50°C to obtain 20mg of light yellow solid powder, yield 23 %. 1 H NMR (300MHz, DMSO-d 6 )δ10.21(s, 1H), 9.54(s, 1H), 8.60(s, 1H), 7.85(d, J=9.4Hz, 2H), 7.81(d, J=9.0Hz, 2H), 7.63( d, J=8.6Hz, 2H), 7.60(d, J=9.5Hz, 1H), 7.40(s, 1H), 5.87(s, 2H), 2.38(t, J=7.3Hz, 2H), 1.63( p, J=7.1Hz, 2H), 1.26(s, 26H), 0.90-0.84(m, 3H). HRMS (ESI): found557.35957 (C 33 h 44 N 6 o 2 [M+H] + , requires557.35985).
Embodiment 2
[0042]N-(6-(1H-imidazol-1-yl)imidazo[1,2-a]pyridin-2-yl)-4-(2-(2-(2-(palmitoylaminoethoxy)ethyl Preparation of oxy)acetamido)benzamide (DDO-3702).
[0043] synthetic route:
[0044]
[0045] (1) N-(6-(1H-imidazol-1-yl)imidazo[1,2-a]pyridin-2-yl)-4-(2-(2-(2-(2-palmitoylamino Preparation of ethoxy)ethoxy)acetamido)benzamide (3).
[0046] Dissolve compound 2 (500mg, 1.25mmol) in 15ml of thionyl chloride, add 2 drops of N,N-dimethylformamide, and reflux at 80°C for 3 hours; after concentrating the reaction solution, add 5ml of N,N-dimethylformamide Amide, make the N, N-dimethylformamide solution of the acid chloride for subsequent use; under ice-cooling, add the N, N-dimethylformamide solution (5ml) of the acid chloride dropwise under ice-cooling N,N-dimethylformamide solution, react overnight at room temperature; add saturated sodium bicarbonate solid to adjust the pH of the reaction solution to alkaline; concentrate the reaction solution, column chromatography purification...
Embodiment 3
[0050] N-(6-(1H-imidazol-1-yl)imidazo[1,2-a]pyridin-2-yl)-4-(6-palmitoylaminocaproylamido)benzamide (DDO-3703) preparation.
[0051] synthetic route:
[0052]
[0053] (1) tert-butyl (6-((4-((6-(1H-imidazol-1-yl)imidazo[1,2-a]pyridin-2-yl)carbamoyl)phenyl)amino) - Preparation of 6-oxyhexyl) carbamate (4).
[0054] Dissolve tert-butoxycarbonyl 6-aminocaproic acid (727mg, 3.14mmol) and N,N'-carbonyldiimidazole (510mg, 3.14mmol) in 6ml of anhydrous N,N-dimethylformamide, ice-bath , stirred for 30 minutes; compound 1 (1g, 3.14mmol) was added dropwise in N,N-dimethylformamide solution, and reacted at room temperature for 4 hours; the reaction solution was concentrated and purified by column chromatography (eluent: ethyl acetate) ; Obtain 100 mg of yellow solid powder, yield 6%. 1 H NMR (300MHz, DMSO-d 6 )δ10.24(s, 1H), 9.45(s, 1H), 8.16(s, 1H), 7.81(d, J=8.4Hz, 2H), 7.70(s, 1H), 7.64(d, J=8.5 Hz, 4H), 7.56(d, J=9.4Hz, 1H), 7.16(s, 1H), 6.84(s, 1H), 5.87(s, 2H), 2.94(q, J=7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com