High-stability inactivated virus preserving fluid and preparation method thereof
A high-stability, preservation solution technology, applied in the medical field, can solve problems such as the integrity of virus nucleic acid needs to be improved, nucleic acid damage, etc., to achieve the effect of improving protection efficiency, improving research utility, and ensuring integrity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
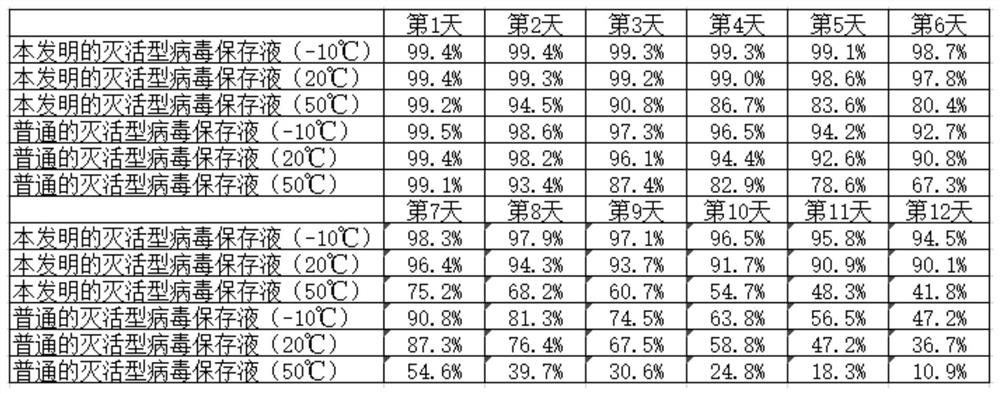
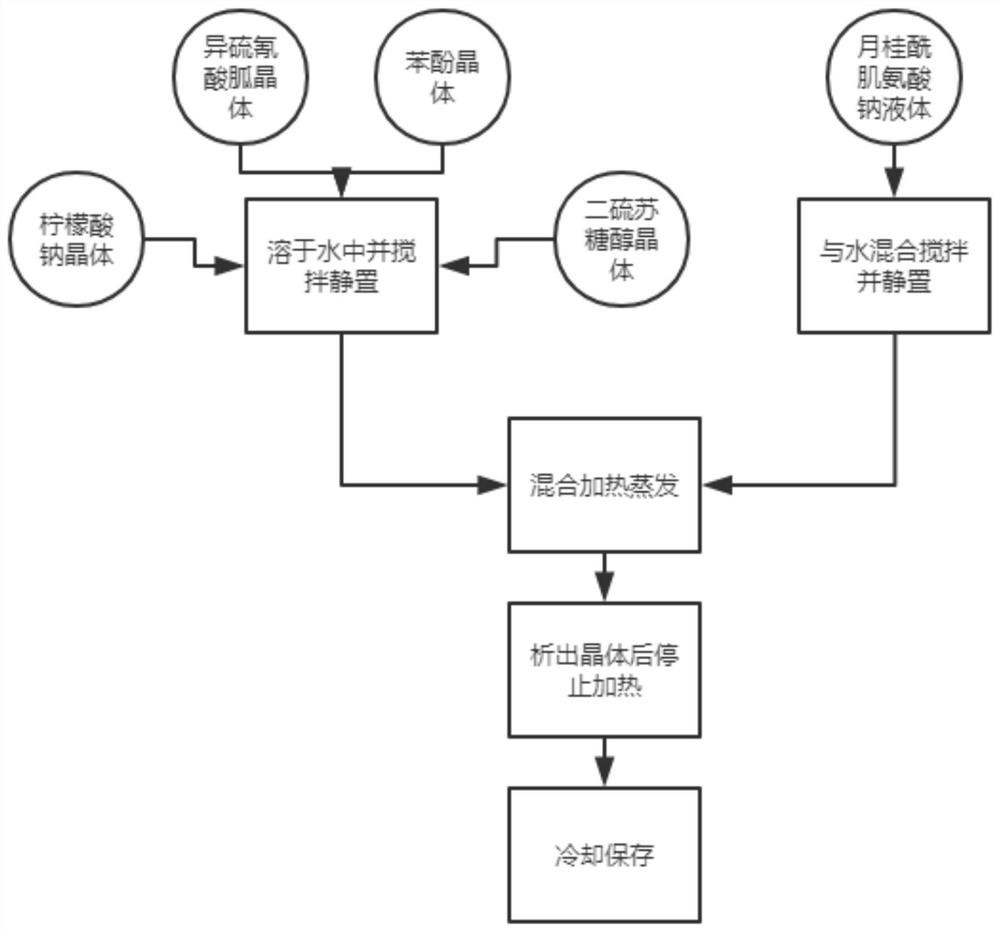
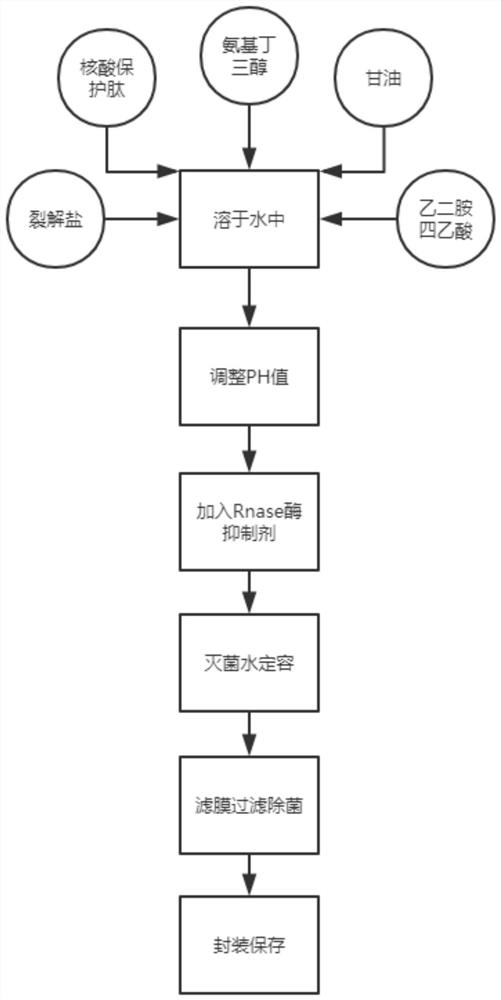
[0026] A highly stable inactivated virus preservation solution, including lysis salt, RNase enzyme inhibitor, tromethamine, ethylenediaminetetraacetic acid, glycerol, nucleic acid protection peptide, water, and the RNase enzyme inhibitor includes iso Guanidine Thiocyanate, Phenol, Sodium Citrate, Dithiothreitol, and Sodium Lauroyl Sarcosinate;
[0027] The weight ratio of each component in the RNase enzyme inhibitor is: 16 parts of guanidine isothiocyanate, 2 parts of phenol, 10 parts of sodium citrate, 1 part of dithiothreitol, and 1 part of sodium lauroyl sarcosinate;
[0028] The protection solution is composed of the following proportions by weight: including 9 parts of cleavage salt, 20 parts of RNase inhibitor, 14 parts of tromethamine, 3 parts of ethylenediaminetetraacetic acid, 10 parts of glycerin, 6 parts of nucleic acid protection peptide, 100 parts of water.
Embodiment 2
[0030] A highly stable inactivated virus preservation solution, including lysis salt, RNase enzyme inhibitor, tromethamine, ethylenediaminetetraacetic acid, glycerol, nucleic acid protection peptide, water, and the RNase enzyme inhibitor includes iso Guanidine Thiocyanate, Phenol, Sodium Citrate, Dithiothreitol, and Sodium Lauroyl Sarcosinate;
[0031] The weight ratio of each component in the RNase enzyme inhibitor is: 16 parts of guanidine isothiocyanate, 2 parts of phenol, 10 parts of sodium citrate, 1 part of dithiothreitol, and 1 part of sodium lauroyl sarcosinate;
[0032] The protection solution is composed of the following proportions by weight: including 9 parts of cleavage salt, 20 parts of RNase inhibitor, 14 parts of tromethamine, 3 parts of ethylenediaminetetraacetic acid, 10 parts of glycerin, 6 parts of nucleic acid protection peptide, 100 parts of water;
[0033] The method for preparing the above-mentioned inactivated virus preservation solution is characteri...
Embodiment 3
[0038] A highly stable inactivated virus preservation solution, including lysis salt, RNase enzyme inhibitor, tromethamine, ethylenediaminetetraacetic acid, glycerol, nucleic acid protection peptide, water, and the RNase enzyme inhibitor includes iso Guanidine Thiocyanate, Phenol, Sodium Citrate, Dithiothreitol, and Sodium Lauroyl Sarcosinate;
[0039]The weight ratio of each component in the RNase enzyme inhibitor is: 16 parts of guanidine isothiocyanate, 2 parts of phenol, 10 parts of sodium citrate, 1 part of dithiothreitol, and 1 part of sodium lauroyl sarcosinate;
[0040] The protection solution is composed of the following proportions by weight: including 9 parts of cleavage salt, 20 parts of RNase inhibitor, 14 parts of tromethamine, 3 parts of ethylenediaminetetraacetic acid, 10 parts of glycerin, 6 parts of nucleic acid protection peptide, 100 parts of water;
[0041] The method for preparing the above-mentioned inactivated virus preservation solution is characteriz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com