Brucellosis nucleic acid vaccine
A kind of Brucella, nucleic acid vaccine technology, applied in the field of Brucella nucleic acid vaccine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1, the preparation of brucella vaccine
[0034] 1. Construction of eukaryotic expression vector:
[0035] The BCSP31, sodC and L7 / L12 genes of Brucella were amplified by PCR and cloned into the eukaryotic expression vector pJW4303 for expression in eukaryotic cells. GenBank accession numbers are: BCSP31: M20404; sodC: NC_007624; L7 / L12: L19101. The genomic DNA of Brucella abortus 2308 (purchased from China National Institute for the Control of Pharmaceutical and Biological Products) extracted by conventional methods was used as a template, and the BCSP31, sodC and L7 / L12 genes were amplified by PCR and cloned into the tissue on the pJW4303 vector Downstream of the plasminogen activator (TPA) signal sequence, a fusion protein is formed. The primer sequence used to amplify BCSP31 is as follows: 5'-GCTAGCCATATGAAATTCGGAAGCAAAATCCGTC-3' and 5'-CGGGATCCTTATTTCAGCACGCCCGCTTCC-3'; the primer sequence used to amplify sodC is as follows: 5'-GCTAGCCATATGAAAAAACGGCTT-3'...
Embodiment 2
[0037] Embodiment 2, identification of Brucella nucleic acid vaccine expressed in vivo
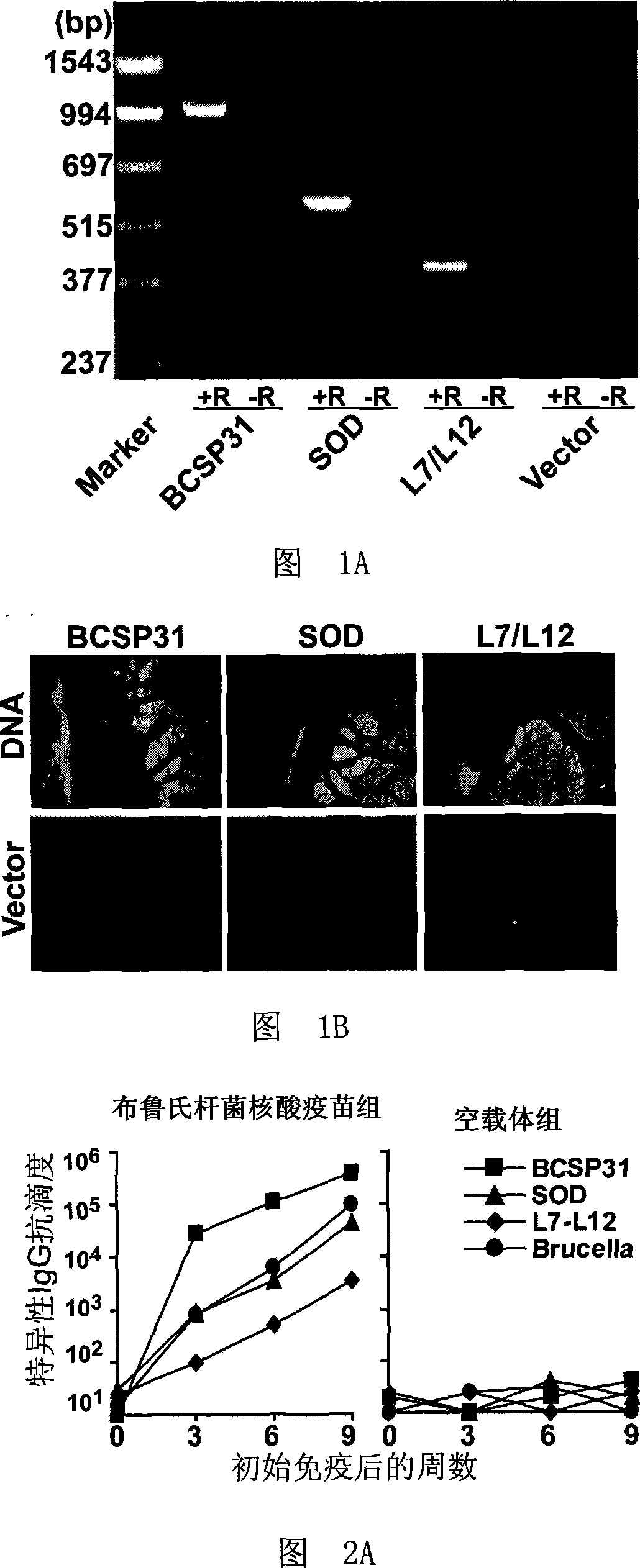
[0038] 1. RT-PCR identification of the expression of BCSP31, sodC and L7 / L12 genes
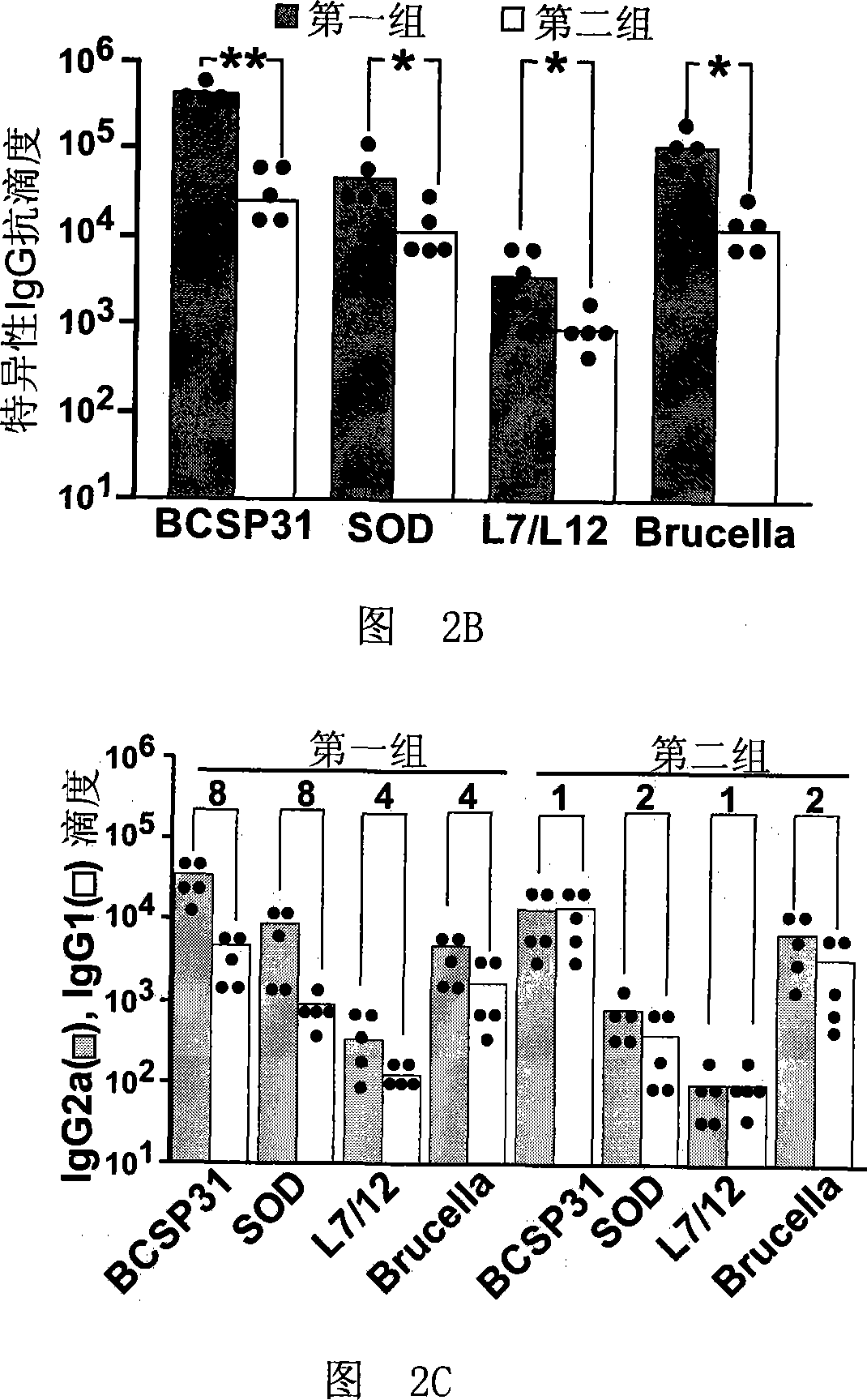
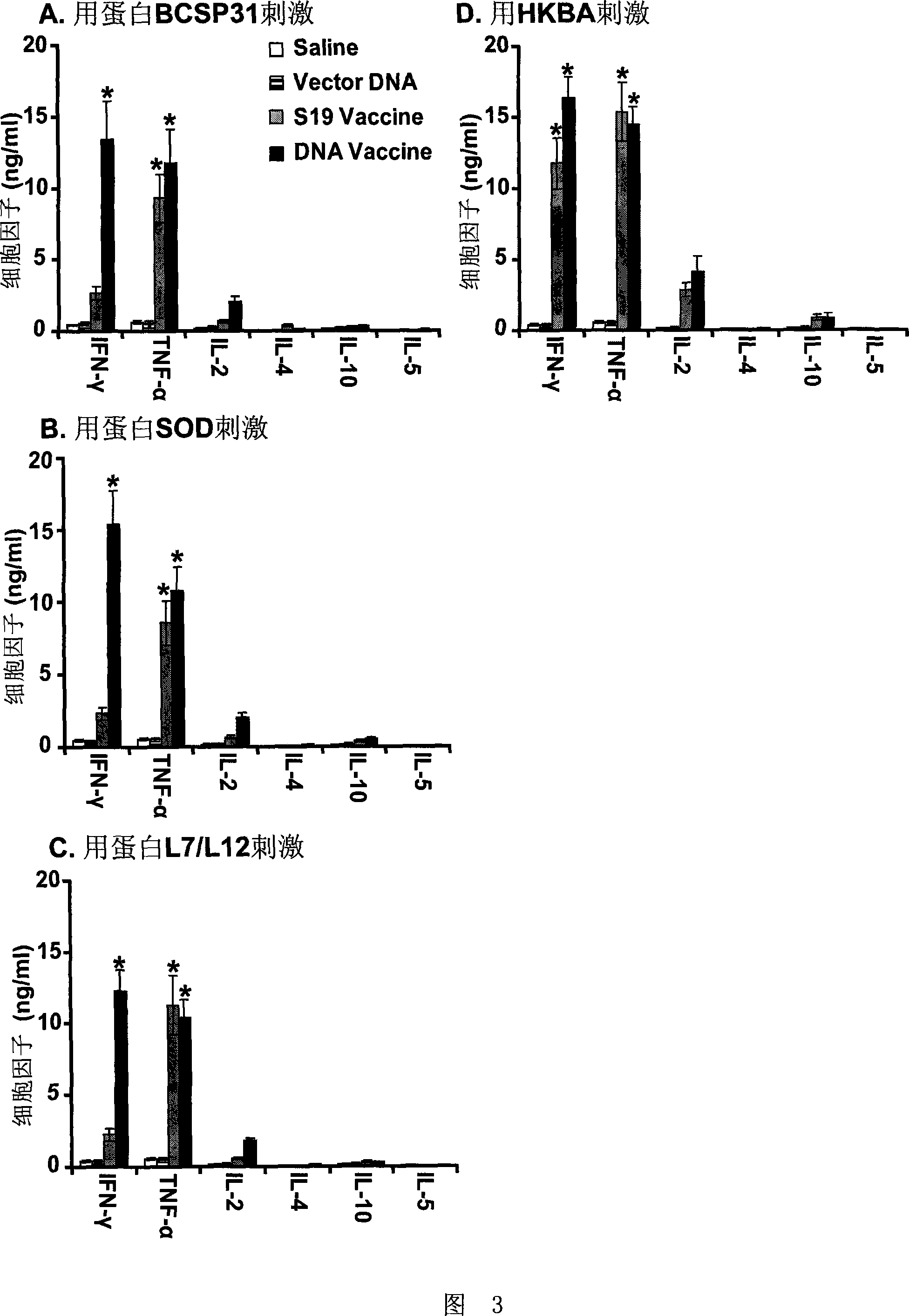
[0039] 20 C57BL / 6 mice were equally divided into two groups, and were treated as follows: the first group was the non-immune group (control), and each mouse was injected intramuscularly with 150 μl saline containing 150 μg pJW4303; the second group was treated with Brucella For the nucleic acid vaccine group, 50 μg of each of the plasmids pJBCSP31, pJsodC and pJL7 / L12 prepared in Example 1 were dissolved in 150 μl of normal saline and mixed evenly to obtain the Brucella nucleic acid vaccine. The brucella nucleic acid vaccine was immunized by intramuscular injection, and the immunization dose was 150 μl / bird. Four weeks after the completion of the immunization, spleens of 10 mice were taken from each group, and their total RNA was extracted using Trizol reagent (Gibco, USA). Then it was reverse transcribe...
Embodiment 3
[0045] Embodiment 3, the immune effect experiment of brucella nucleic acid vaccine:
[0046] 60 C57BL / 6 mice were equally divided into 4 groups, and were processed as follows: the first group was the Brucella nucleic acid vaccine group, each 50 μg of plasmids pJBCSP31, pJsodC and pJL7 / L12 prepared in Example 1 were dissolved together. In 150 μl of physiological saline, mix well to obtain the Brucella nucleic acid vaccine. The mice were immunized by intramuscular injection three times, the immunization dose was 150 μl / mouse / time, and the time points were the 0th week, the 3rd week, and the 6th week. The second group is the S19 vaccine group, 1×10 9 CFU S19 freeze-dried protein (purchased from China National Institute for the Control of Pharmaceutical and Biological Products, No. 040611, approval number: Xinxin Yaozi (2001) 287710) was dissolved in 150 μl of normal saline to obtain the S19 vaccine; the mice were immunized by intramuscular injection three times, and the immune ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com