Pteridinone compound and application thereof
A kind of compound, the technology of pteridone, which is applied in the field of pteridone compounds and achieves remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
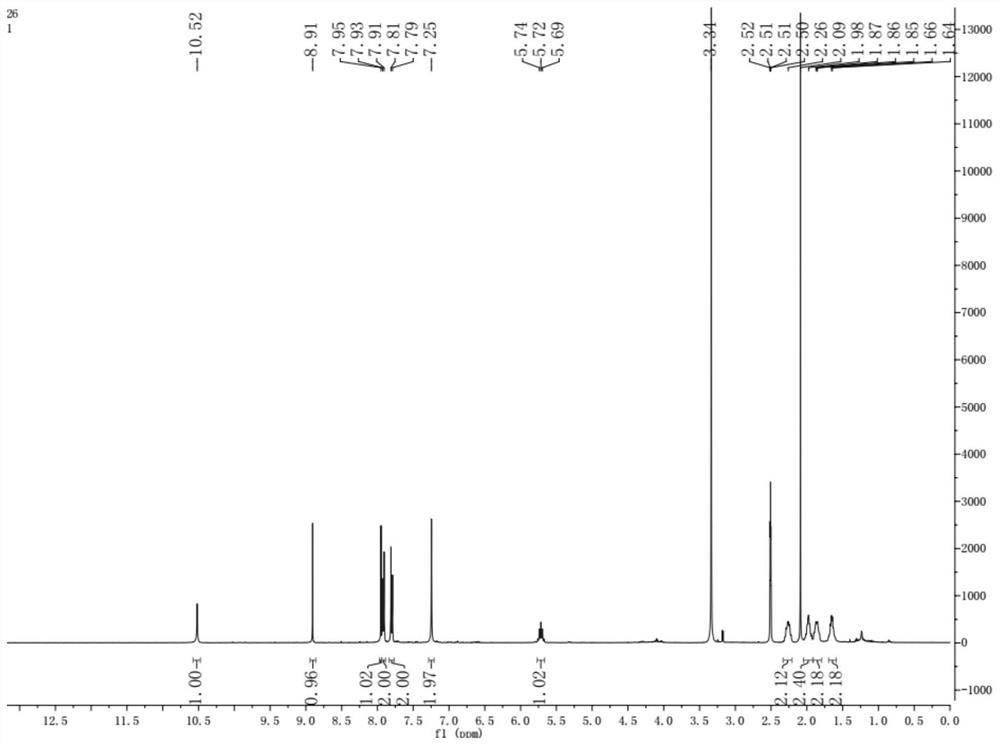
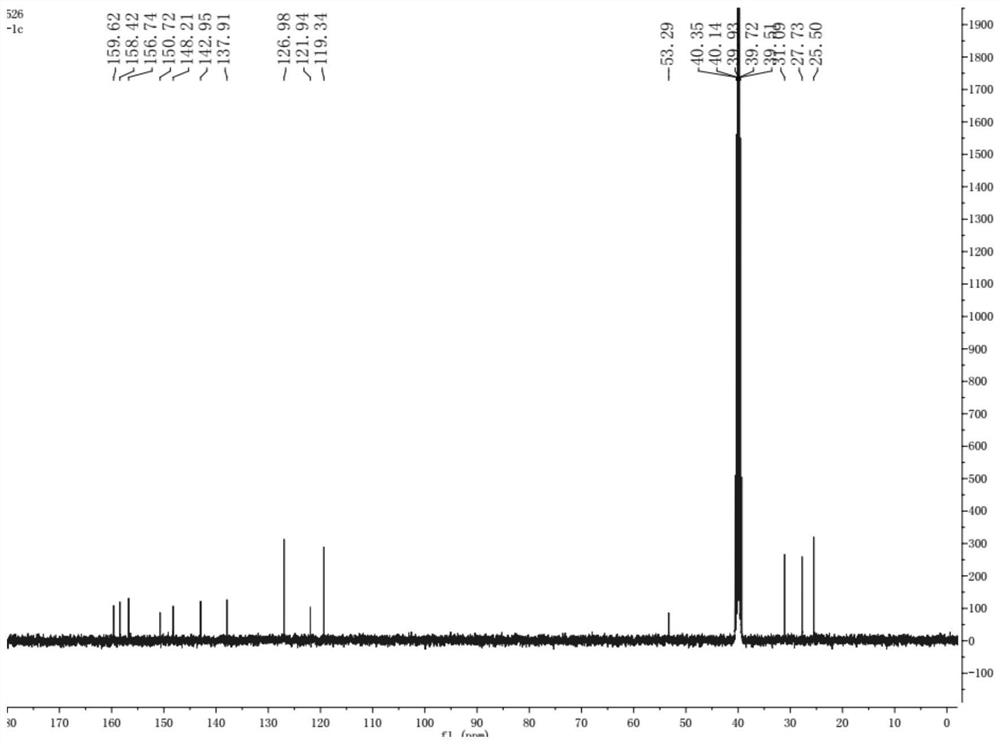
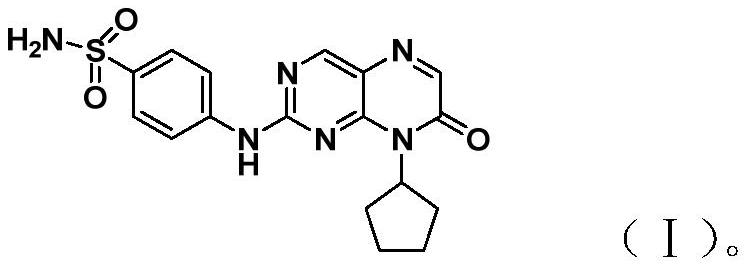
[0020] Example 1 [Preparation 4- (8-cyclopentyl-7-oxo-7,8-dihydrobin-2-yam) -benzenesulfonamide]
[0021] (1) 4.0 mmol of 2,4-dichloro-5-nitrpyrimidine, 4.2 mmol of potassium carbonate was added to 30 ml of acetone, stirred, and after 10 minutes, 4.0 mmol cyclopentamine, TLC was monitored, and the reaction was completed. Afterward, the reaction solution was poured into 100 ml of water, extracted with ethyl acetate (100 mL × 3), washed withide, and washed brine (50 mL × 3) with aqueous sulfate, then dried over anhydrous magnesium sulfate. Ethyl acetate is removed under reduced pressure 2-chloro-N-cyclopentyl-5-nitrimidine-4-amine; the chemical reaction form of this step see the chemical formula (IV) in the present invention;
[0022] (2) 2.0 mmol of step (1) of the intermediate, 2.1 mmol of potassium carbonate was added to 20 mL of ethanol, stirred, and after 10 minutes, 2.0 mmol was added to aminobenzenesulfonamide, TLC monitoring, and after completion of the reaction, The reactio...
Embodiment 2
[0026] Example 2 (Research on Antioma Activity)
[0027] The in vitro anti-tumor activity of the compound of the present invention is verified using the following method. The following effects indicate that the compounds of the invention can be used to treat cancer, particularly the treatment of solid tumors, such as cervical cancer, colon cancer, and breast cancer. The specific verification method is as follows:
[0028] Summary antitumor activity of the butterfly compound prepared by the 1 MTT method was used. Collecting the alternative cell, adjusting the concentration of cell suspension, with 3 × 10 3 -4 × 10 3 The / ml was inoculated on 96-well plates, incubated 12-24h. After the cells were attached to the cell, different concentrations of drugs were added, and 0.1, 0.3, 1, 3, 10, 30 μmol / L total 6 concentration gradients were set, each concentration of 4 complex apertures. Place 37 ° C, 5% CO 2 The incubator and start timing culture. The 96-well plate was taken out after d...
Embodiment 3
[0035] Example 3 (CDK4, CDK6 enzyme inhibitory activity)
[0036] (1) Preparation of drugs: Formulated by DMSO to 10 mm mother liquor, ultrasonic accelerated dissolution, and then diluted with DMSO and kinase buffer, ensuring that the final concentration of DMSO is less than 1%;
[0037] (2) Ingredients of kinase reaction buffer: 40 mM Tris, pH 7.4, 10 mM mgCl2, 0.1 mg / ml BSA, 1MMDTT, 10 μM ATP;
[0038] (3) Detailed experimental steps are: On white enzyme board, add 1 ul of drugs per well, then add 10 μl CycLin D3 enzyme to mix, add 5 μL of Histone H1 substrate, then add 34 μl of analysis buffer, mixed, 40 minutes at 30 ° C, then adding 50 μl of ATP detection solution for 5 minutes, immediately detecting a chemiluminescent signal on the enzyme gauge, the enzyme activity size and chemical light emitting number is inversely proportional, will The value is substituting the following formula, calculating the percentage of activity:
[0039] % Activity = {(LU Drug - L. bottom) / (lu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com