A hyaluronic acid-docetaxel conjugate modified by dendrimers and its preparation method
A technology of docetaxel and hyaluronic acid, which is applied in the field of hyaluronic acid-docetaxel conjugates, can solve the problems of short residence time, high toxicity and side effects, and poor water solubility, so as to improve the therapeutic effect, prolong the circulation time, The effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
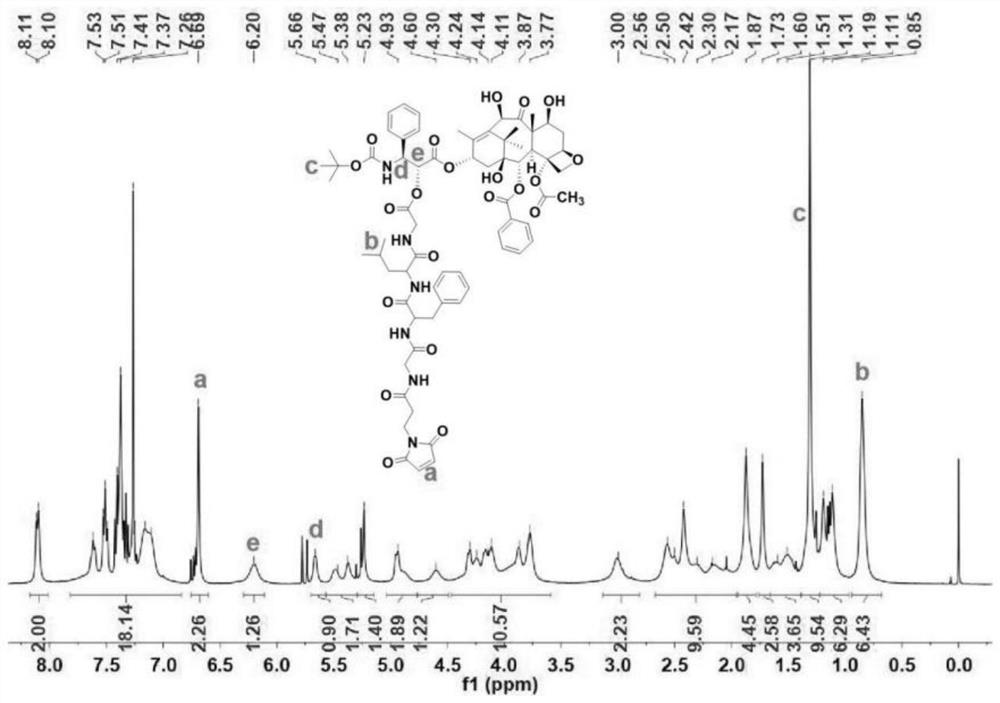
[0041] A dendrimer-modified hyaluronic acid-docetaxel conjugate, the conjugate is hyaluronic acid loaded with docetaxel and a dendrimer, and the structure is as follows:
[0042]
[0043] Wherein, n is selected from 0, 1, 2, 3, 4, 5....
Embodiment 2
[0045] This embodiment is on the basis of embodiment 1:
[0046] The particle size of the conjugate is 118-126nm.
[0047] The molecular weight of the hyaluronic acid is 48000Da.
Embodiment 3
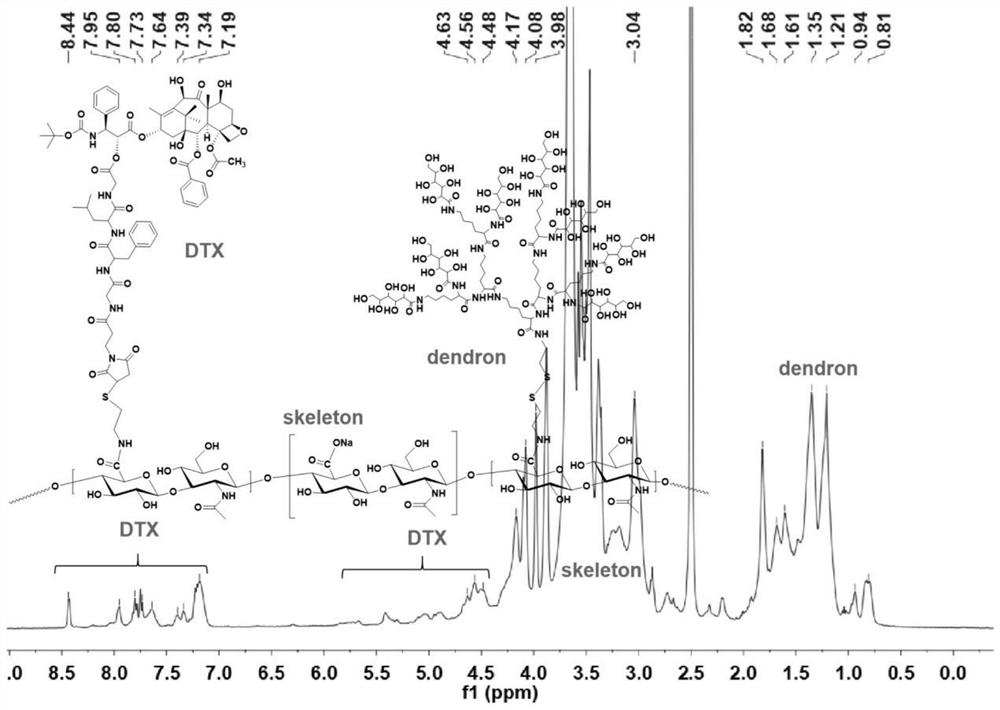
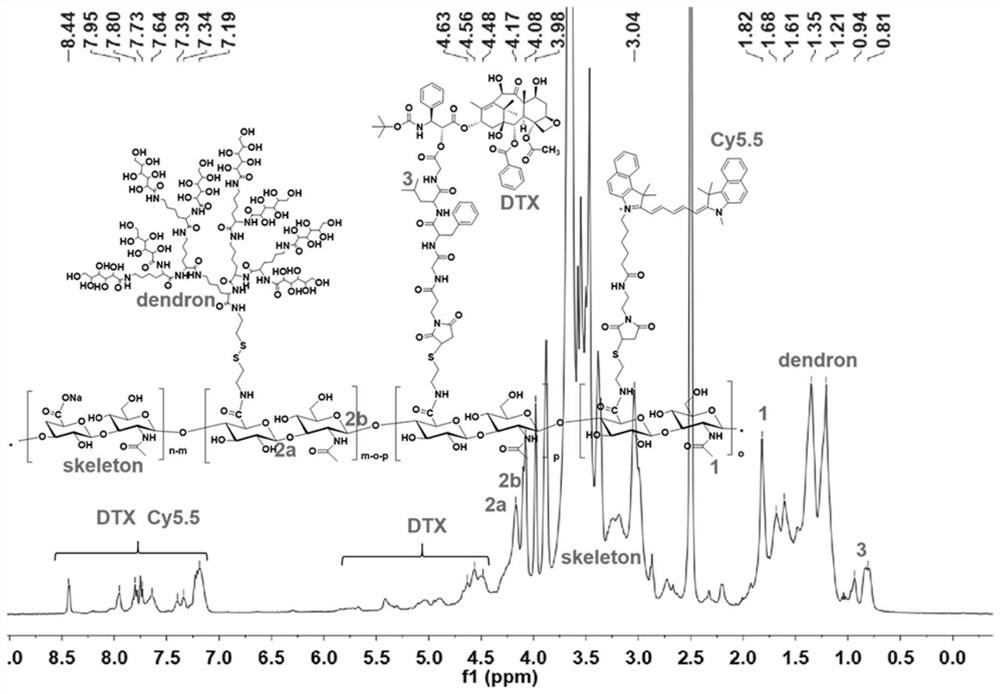
[0049]Synthesis of dendrimer-modified hyaluronic acid-docetaxel conjugates:
[0050]
[0051] Introduce aminoethanethiol on the hyaluronic acid as the carrier to obtain HA-SH for later use; couple the maleimide-modified GFLG with docetaxel to obtain the conjugate Mal-GFLG-DTX, and then pass the thiol -Ene click reaction to connect Mal-GFLG-DTX to HA-SH to obtain a conjugate intermediate; then connect the saccharified dendron-8Glu to the sulfhydryl group on hyaluronic acid through sulfhydryl exchange reaction to obtain the finished product .
[0052] The synthesis method of HA-SH and Dendron-8Glu refers to "Glycodendron / pyropheophorbide-a(Ppa)-functionalized hyaluronic acid as a nanosystem for tumor photodynamic therapy" (Carbohydrate Polymers, 2020, 247, 116749).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com