Amphiphilic triblock copolymer, preparation method and siRNA drug carrier
A hydrophilic polymer and polymer technology, applied in the field of life medicine, can solve the problems of low transfection efficiency, immune stimulation, toxicity, etc., and achieve the effect of high repeatability, good biocompatibility, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1, the synthesis and characterization of prepolymer mA-B-acrylated and mB-A-acrylated
[0063] 1. Synthesis and characterization of propylene-terminated polyhydroxyalkanoate monomethyl ether-polyethylene glycol (mA-B-acrylated)
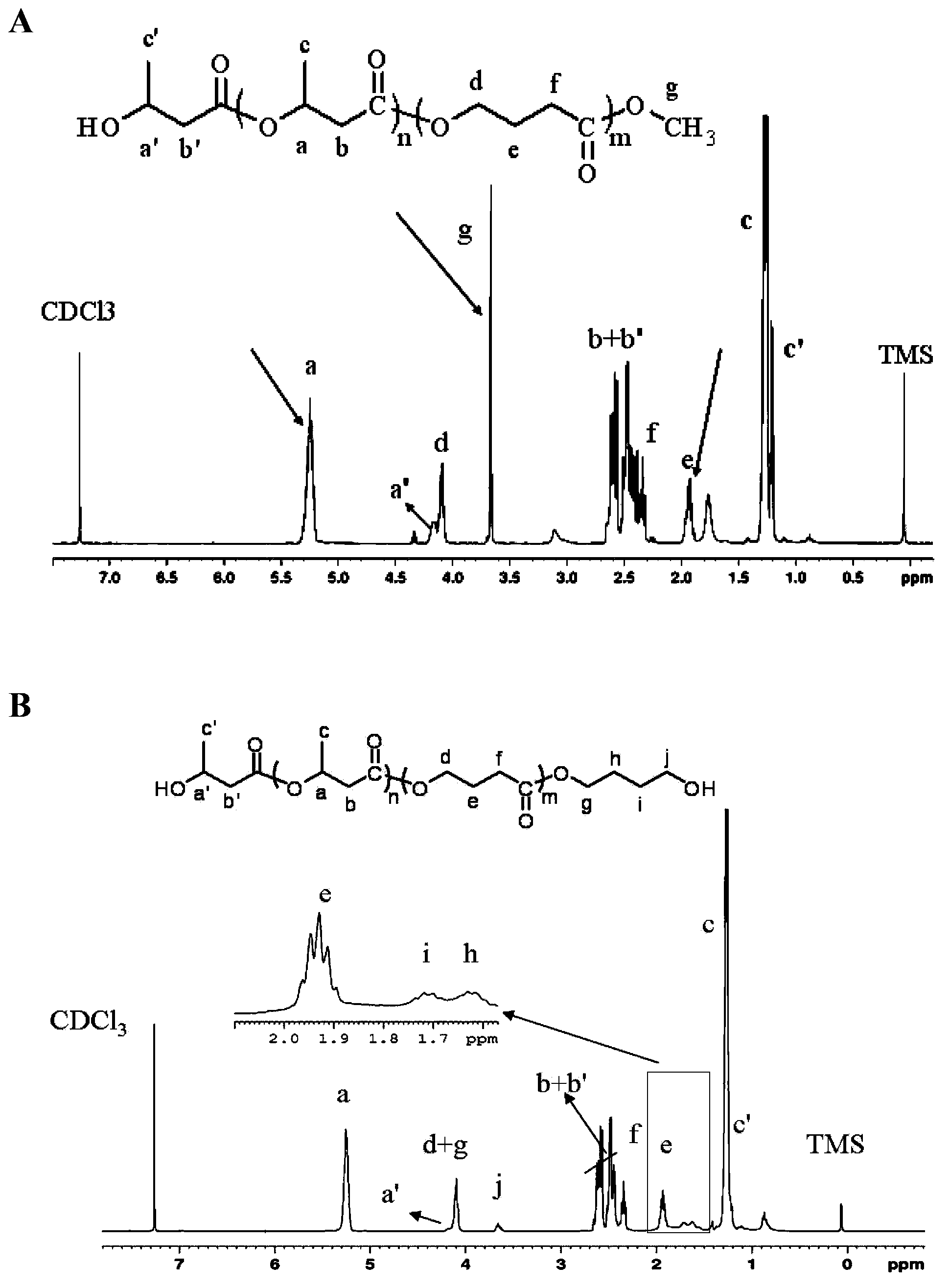
[0064] (1) Synthesis and characterization of mono / dihydroxy polyhydroxyalkanoate (mPHA-OH / PHA-diol)
[0065] The synthesis of various mono / dihydroxy polyhydroxyalkanoate (mPHA-OH / PHA-diol) is prepared by transesterification reaction with methanol or diol under the catalysis of p-toluenesulfonic acid (PTSA). The alcohol may be ethylene glycol, 1,3-propanediol, 1,4-butanediol, 1,6-hexanediol. All kinds of polyhydroxyalkanoate raw materials in the present invention are purchased from Shenzhen Ecoman Biotechnology Co., Ltd. The diol (P3 / 4HB-diol ) as an example to illustrate the operation steps of this type of reaction.
[0066] The synthetic route of mP3 / 4HB-OH is as follows figure 1 (A) shown. mP3 / 4HB-OH is prepared by transeste...
Embodiment 2
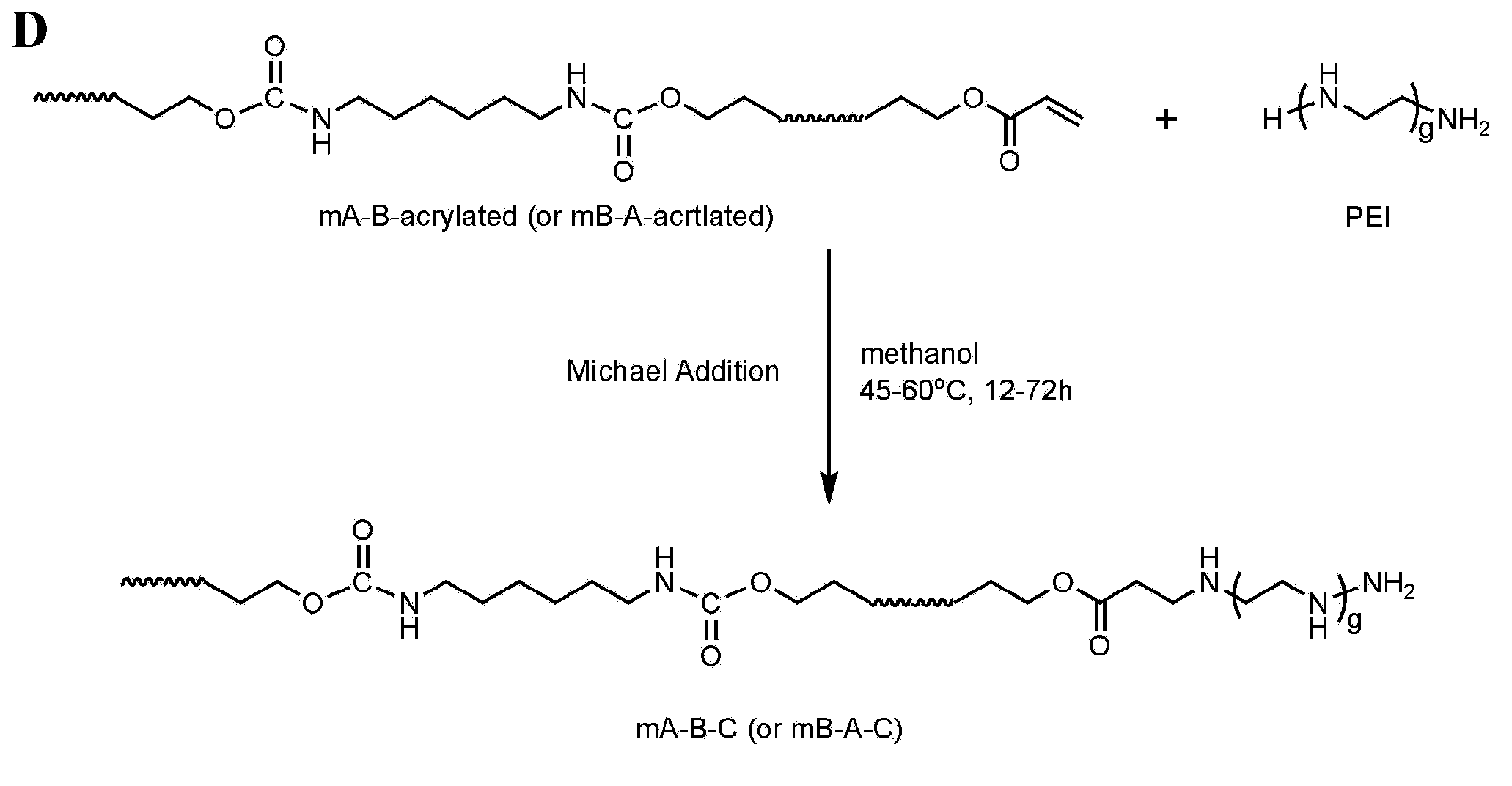
[0092] Embodiment 2, the synthesis and characterization of formula I compound mA-B-C or mB-A-C
[0093] Various types of mA-B-C or mB-A-C copolymers with different molecular weights are made of mA-B-acrylated or mB-A-acrylated with corresponding types and corresponding molecular weights as prepolymers, and react with amino groups of cationic compounds through Michael addition .
[0094] Synthesis schematic as figure 1 As shown in (D): react the terminal acryl group of the prepolymer mA-B-acrylate or mB-A-acrylate with the amino group of the cationic compound under certain conditions to obtain the compound of formula I.
[0095] Using mP3 / 4HB-PEG-acrylate or mPEG-P3 / 4HB-acrylate with cationic polymer lPEI (which has a weight average molecular weight ranging from 600 to 22,000 Daltons, those skilled in the art can according to specific needs, in practice Specific selection in the application) to prepare mP3 / 4HB-PEG-lPEI or mPEG-P3 / 4HB-lPEI copolymer as an example to illustrat...
Embodiment 3
[0105] Embodiment 3, prepare nanoparticle with formula I compound mA-B-C or mB-A-C
[0106] 1. Preparation of nanoparticles
[0107] Amphiphilic triblock copolymers have hydrophobic and hydrophilic interactions in aqueous solution, and nanoparticles can be formed as long as the copolymer concentration is higher than the critical micelle concentration. All kinds of compounds of formula I prepared in the present invention are composed of cationic compound of hydrophilic part, hydrophilic polymer and polyhydroxyalkanoate of hydrophobic part, so they can self-assemble to form nanoparticles in aqueous solution. The following takes the preparation of nanoparticle of material 1 as an example to illustrate the preparation method of various compound nanoparticles of formula I in the present invention.
[0108] There are many ways to prepare nanoparticles, the most common one is the solvent evaporation method. The specific method is: dissolve 20mg of material 1 in 2ml of tetrahydrofura...
PUM
| Property | Measurement | Unit |
|---|---|---|
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
| number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com