Related substance for synthetic building block of Afoxolaner and Fluralaner and synthetic method thereof
A technology related to substances and compounds, applied in the field of medical technology and drug synthesis, can solve the problems of compound synthesis and purification quality research and pharmacology and toxicology research that have not achieved satisfactory results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
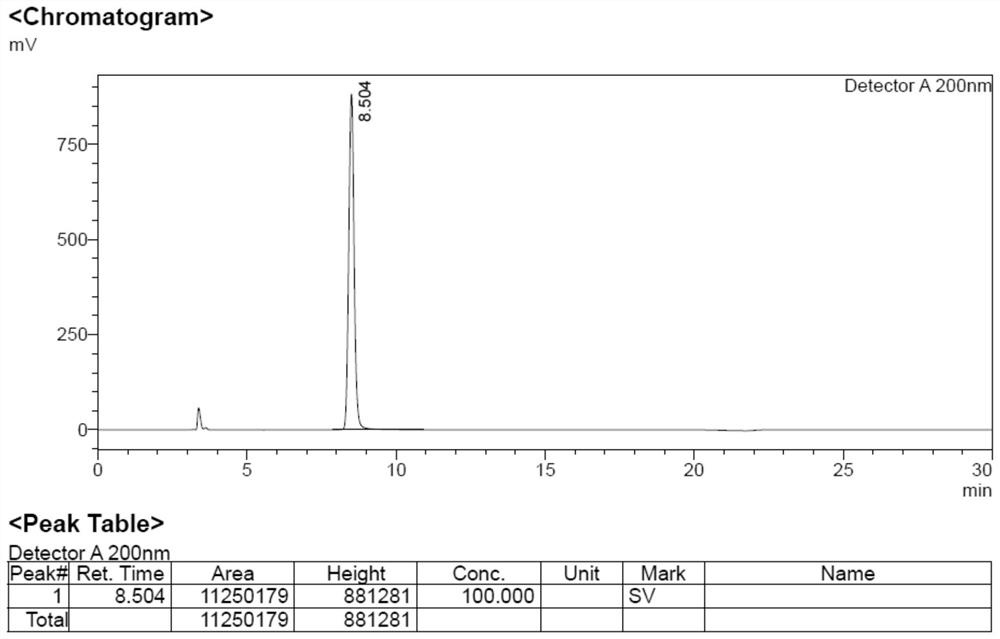
[0040] Add 10 mL of acetic anhydride and 2 g of 2-(1,3-dioxoisoindol-2-yl) acetic acid (Formula 3) into the reaction flask, replace with nitrogen 5 times, stir to raise the temperature to 100°C, and stir for 24 h. Slowly cool to 0°C, slowly add 100mL of water dropwise, stir for 3 hours and filter with suction to obtain a white solid, that is, impurity formula 6: 1.53g, yield 80%.
Embodiment 2
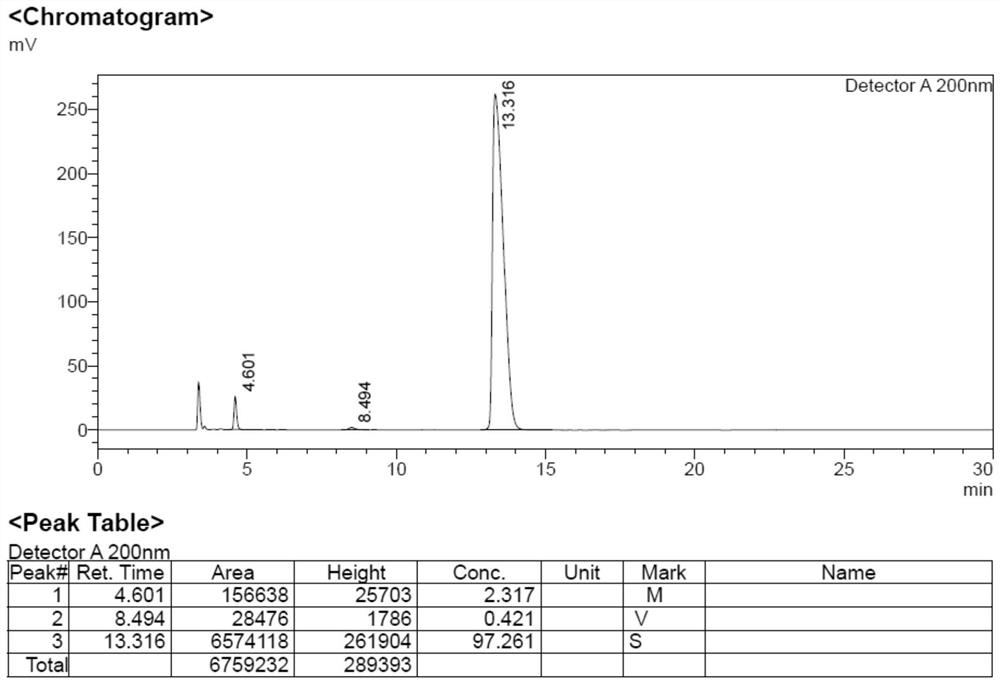
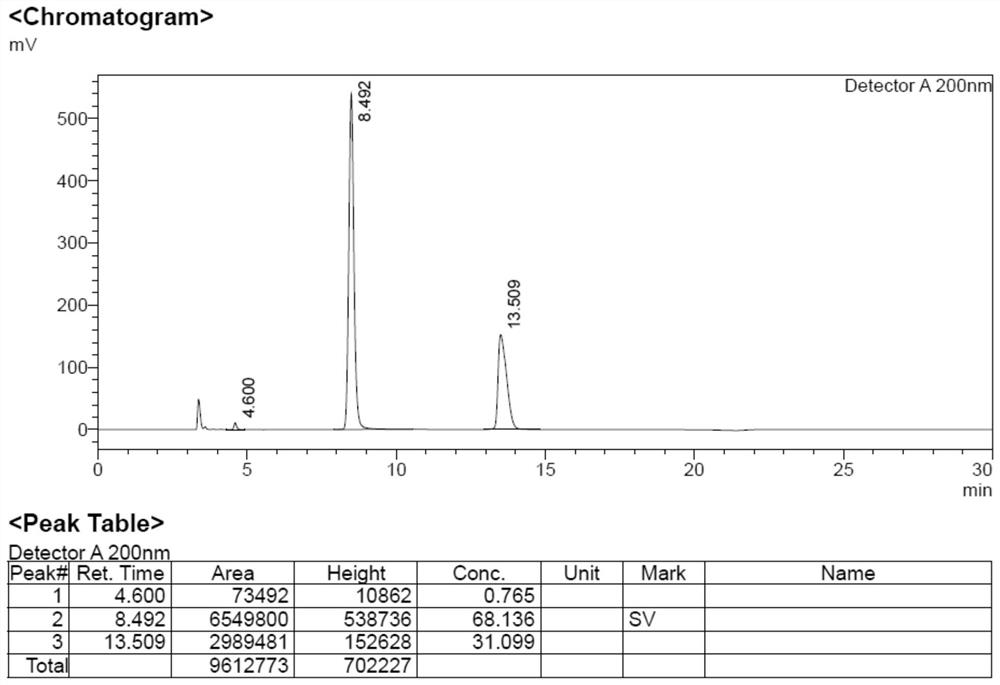
[0042]Add impurity formula 6 (96.7g), hydrazine hydrate (66.6g) and ethanol (300mL) into the reaction flask, heat up to 80°C for reflux reaction for 10h, cool the reaction solution to 45°C, filter with suction, remove insoluble matter, and obtain the filtrate, the filtrate Rotary evaporation under reduced pressure at around 60°C to remove the solvent, concentrate the resulting off-white solid, add 200mL of ethyl acetate to beat for 2h, filter with suction to obtain the filtrate, lower the temperature to 0°C, slowly add 375mL of hydrochloric acid in ethyl acetate solution dropwise, adjust the pH=5~6 , a large amount of white solid was precipitated. Filtrate with suction to obtain a white solid, and dry it in a blast oven at 40°C for 24 hours to obtain the impurity formula 9: 27.3g, with a yield of 42.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com