Tandospirone citrate stable crystal form and preparation method thereof
A technology of tandospirone citrate and tandospirone, which is applied in the field of a stable crystal form of tandospirone citrate and its preparation, and can solve the problems of complex operation, unfriendly environment and high cost of the preparation method problems, to achieve the effects of simplified preparation process, good medicinal safety and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1 Preparation of stable crystalline form of tandospirone citrate
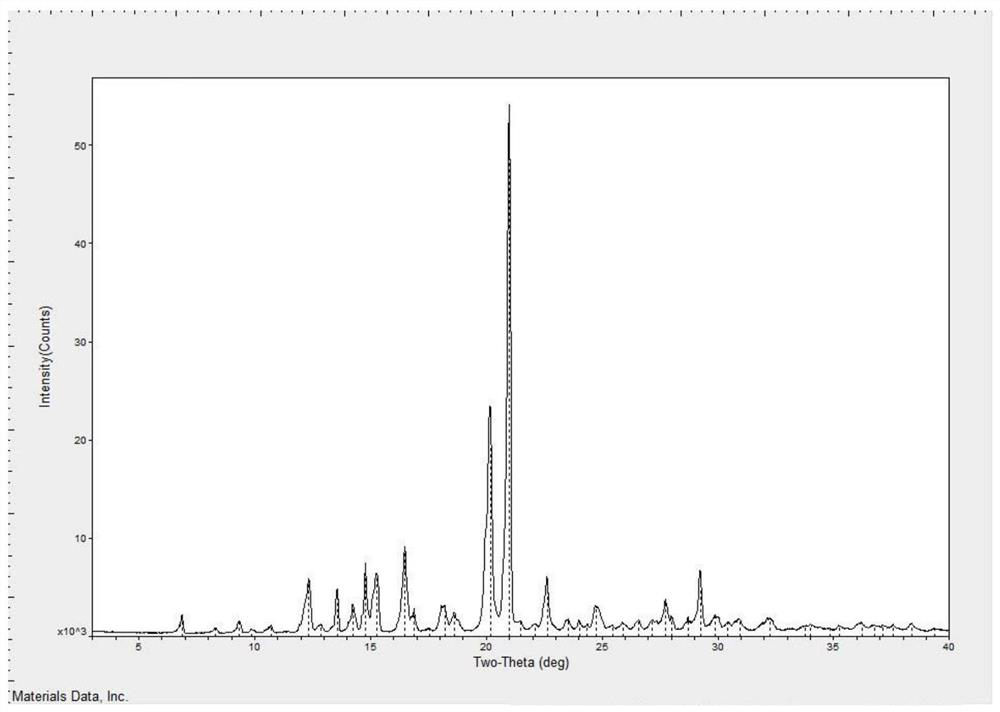
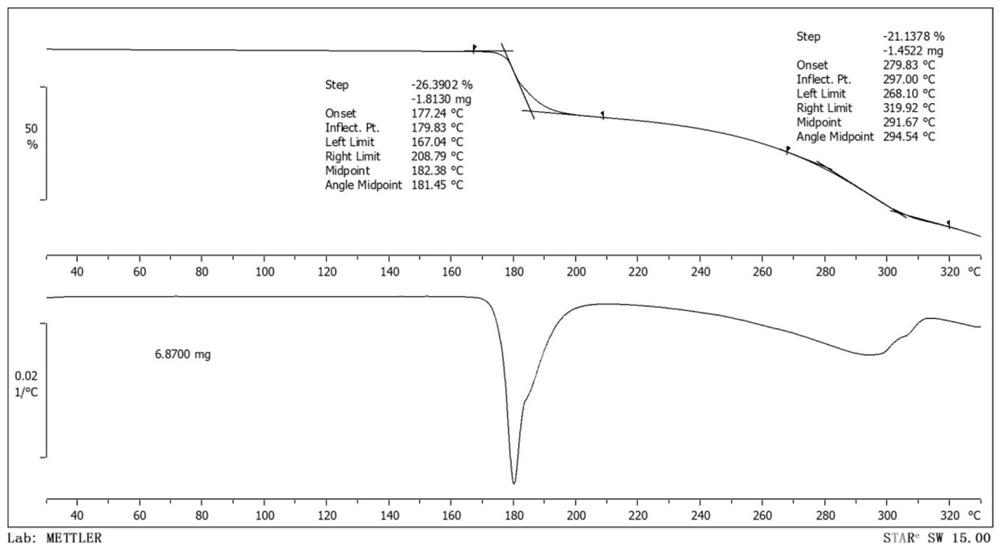
[0032] Weigh 100.0g of tandospirone free base, 50.1g of citric acid, add 1500ml of methanol, stir and heat up to reflux, slowly cool down to 0-20°C, keep stirring for 2 hours, filter, and wash the filter cake with methanol to obtain Citron Tandospirone wet product. Dry the wet product at 65°C for 15 hours to obtain tandospirone citrate with a yield of 88.1% and a purity of 99.89%. The XRPD is as follows: figure 1 , DSC such as figure 2 .
Embodiment 2
[0033] Embodiment 2 Preparation of stable crystalline form of tandospirone citrate
[0034] Weigh 100.0 g of tandospirone free base, 60.1 g of citric acid, add 1500 ml of methanol, stir at room temperature until the solid precipitates, then stir for 2 hours, filter, and wash the filter cake with methanol to obtain the wet product of tandospirone citrate . The wet product was dried at 65°C for 15 hours to obtain tandospirone citrate with a yield of 91.4% and a purity of 99.82%.
Embodiment 3
[0035] Embodiment 3 Preparation of stable crystalline form of tandospirone citrate
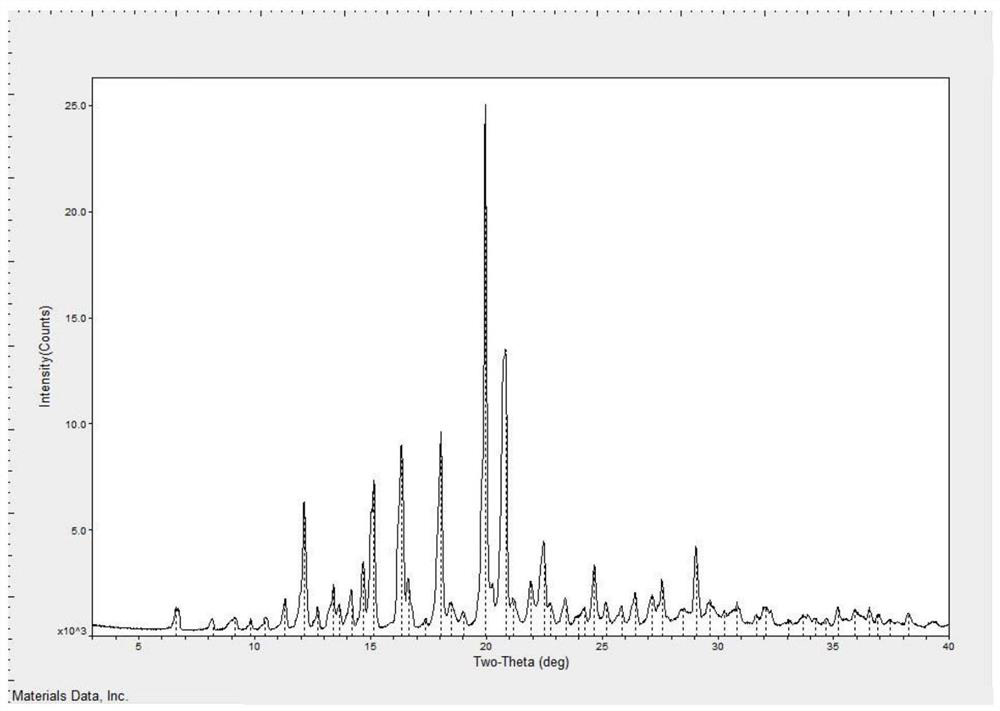
[0036] Weigh 100.0g of tandospirone free base, 60.1g of citric acid, add 1500ml of ethanol, stir and heat up to reflux, slowly cool down to 0-20°C, keep stirring for 2 hours, filter, and wash the filter cake with ethanol to obtain Citron Tandospirone wet product. The wet product was dried at 80°C for 10 hours to obtain tandospirone citrate with a yield of 95.8% and a purity of 99.87%. The XRPD was as follows: image 3 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| diffraction angle | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com