Method for obtaining high-purity heterologous antibody
A high-purity, bispecific antibody technology, applied in the field of obtaining high-purity heterologous antibodies, can solve the problem of high immunogenicity of the heavy chain constant region, achieve broad commercial application prospects, simplify purification steps, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
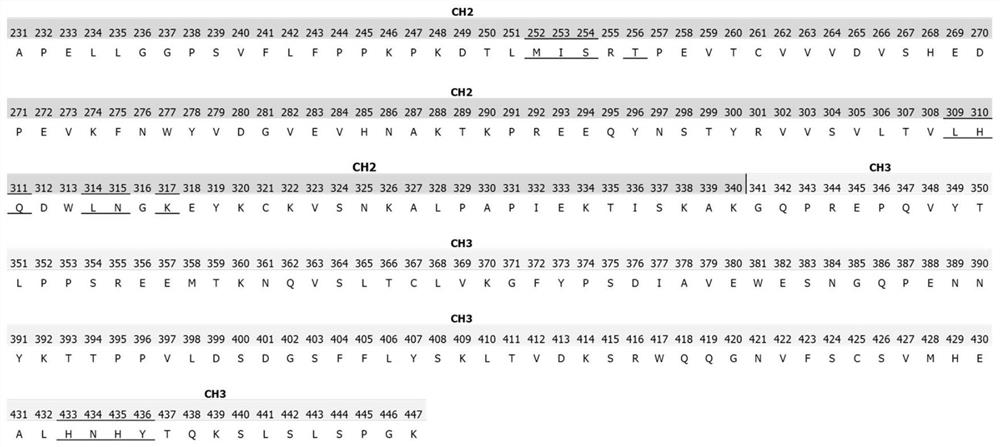
[0058] Example 1. Design of Amino Acid Modifications in the Heavy Chain Constant Region
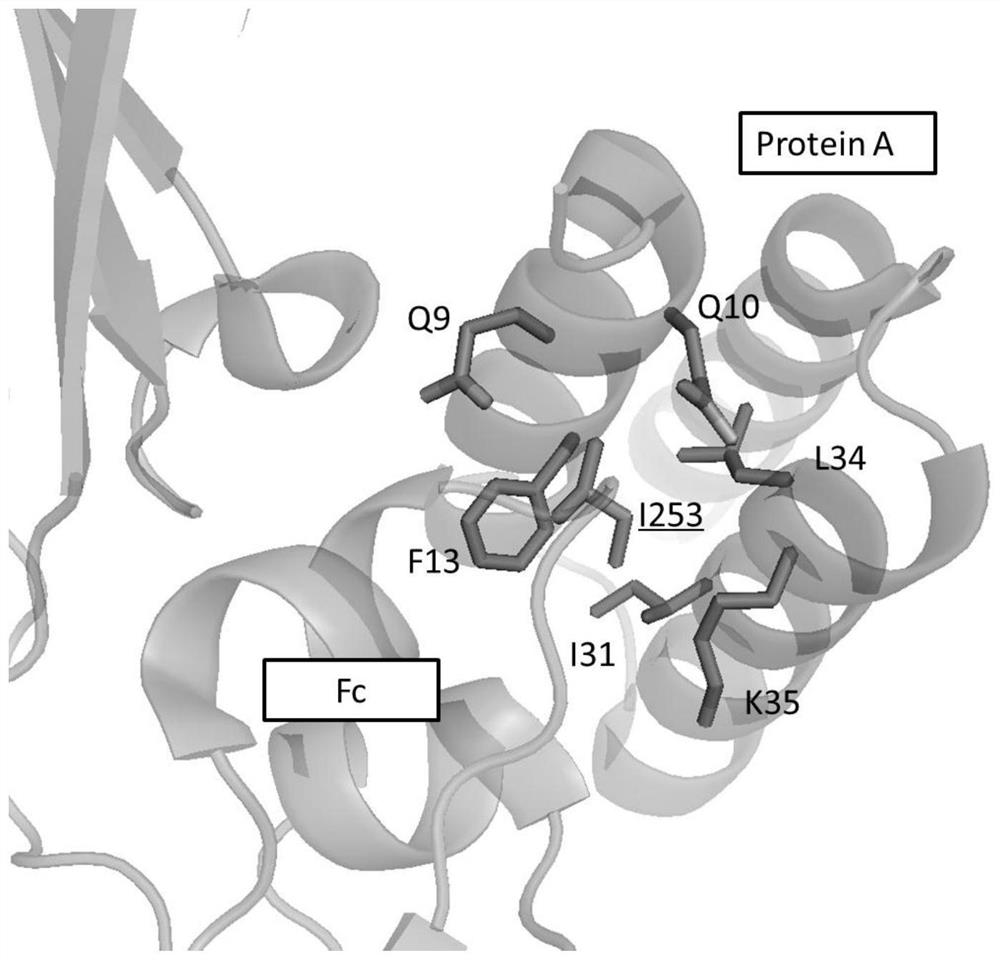
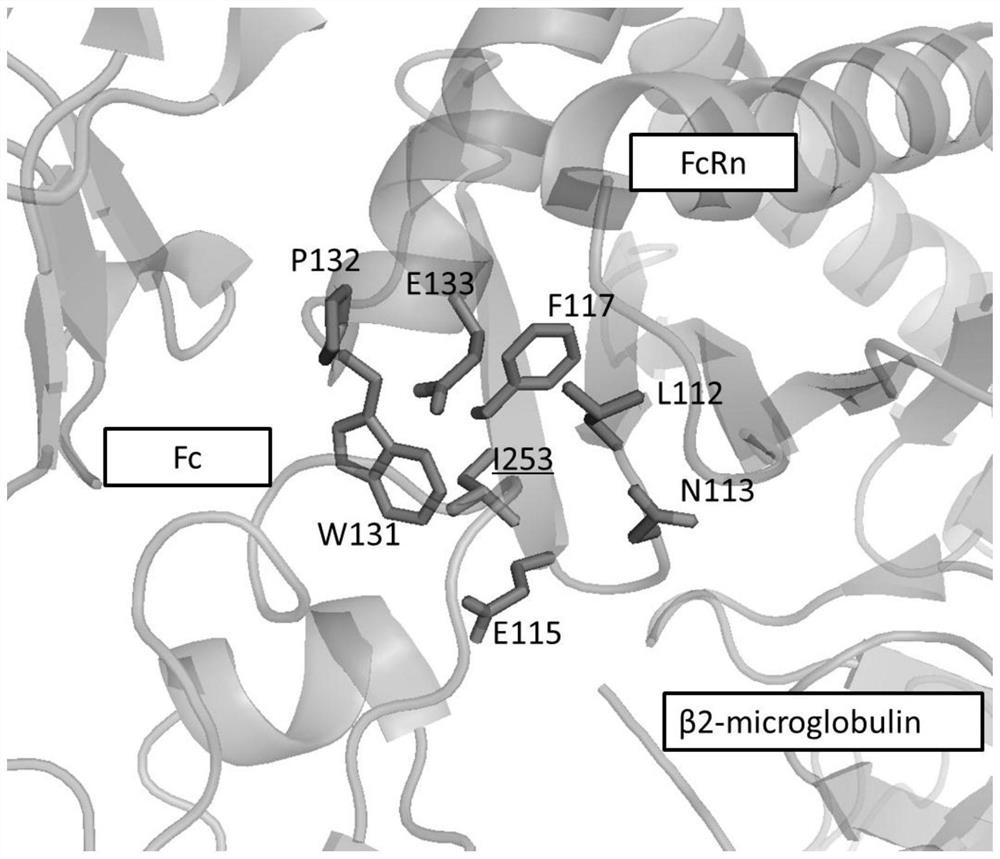
[0059] The binding site of antibody Fc fragment to protein A and FcRn is as follows: figure 1 shown. figure 2 Shows the complex crystal structure of Fc and protein A (PDB code: 4WWI), M252, I253, S254, L309, H310, Q311, L314, N315, K317, H433, N434, H435, Y436 on the Fc segment of the antibody (EU number) on the Fc and protein A interaction interface. image 3 Shows the complex crystal structure of Fc and FcRn (PDB code: 4NOU), M252, I253, S254, T256, L309, H310, Q311, L314, N315, K317, H433, N434, H435, Y436 (EU numbering) are located in Fc and FcRn interaction interface. The present invention creatively proposes that mutating I253 into a positively charged amino acid, such as Lys, Arg, or mutating I253 into a polar amino acid such as Asn, Gln, may make the interaction between Fc and protein A polar- Water repulsion, thereby changing the affinity of Fc for protein A.
Embodiment 2
[0060] Example 2. Construction and expression of antibody expression vectors
[0061] It can be known from Example 1 that mutating the I253 of the heavy chain of the antibody into a positively charged amino acid, such as Lys, Arg, or a polar amino acid such as Asn, Gln, may weaken the interaction between the antibody and protein A, while minimizing Effect on antibody binding to FcRn. Therefore, in this example, a series of PD1×Her2 bispecific antibody molecules with I253 mutation were constructed. Such as Figure 4 As shown, one heavy chain of the bispecific antibody carries the I253 mutation; the other heavy chain does not carry the I253 mutation, and a single-chain antibody fragment is concatenated at the N-terminal of the heavy chain. This allows the molecular weight of the bispecific antibody to be differentiated from the two cognate antibodies. The heterologous antibodies or heterodimers described in this example refer to bispecific antibodies, and the homologous antib...
Embodiment 3
[0070] Example 3. Research on the Elution Conditions of Protein A Affinity Chromatography
[0071] The antibody gene was transfected into 293E cells. After the cells were cultured for 7 days, the culture medium was subjected to high-speed centrifugation and vacuum filtration through a microporous membrane, and then loaded onto a HiTrap MabSelectSuRe column (purchased from GE). The staged use is shown in Table 3 Washing 1, Elution 1-5 The antibody proteins in Table 2 were purified, and the pH was neutralized with Tris buffer at pH 9.0 after elution. The eluted fractions were collected and concentrated, then added to the reduced protein electrophoresis loading buffer and non-reduced protein electrophoresis loading buffer respectively, boiled and then detected by SDS-PAGE.
[0072] table 3
[0073] balance PBS cleaning 1 PBS Elution 1 100mM citric acid, pH5.5 Elution 2 100mM citric acid, pH5.0 Elution 3 100mM citric acid, pH4.5 Elutio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com