Hydrophilic interaction and reversed-phase liquid chromatography coupled to analyze the structure of proanthocyanidins
A reversed-phase liquid chromatography and proanthocyanidin technology, which is applied in the field of rapid analysis of proanthocyanidin structure, can solve the problems of time-consuming and inaccurate proanthocyanidin structure analysis results, and achieve the effect of improving analysis speed and accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0156] Embodiment 1. A method for combined analysis of proanthocyanidin structure by hydrophilic interaction chromatography (Hilic) and reversed-phase liquid chromatography (RPLC), the test sample containing proanthocyanidin is grape seed extract, and the following steps are carried out successively :
[0157] 1), flavan-3-ol monomer analysis:
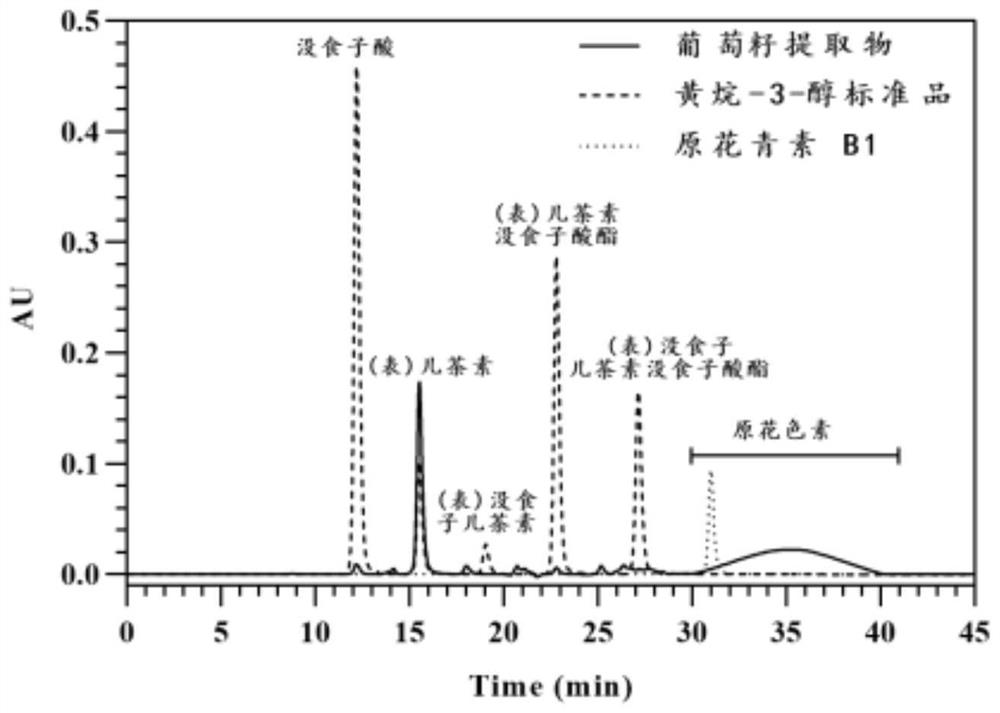
[0158] Hilic analyzes (table) catechins and their gallates in the sample solution to be tested, as follows:
[0159] Accurately weigh 5.0 mg of grape seed extract, dissolve it in 1.0 mL of 97% acetonitrile aqueous solution, and use it as the sample solution to be tested; after passing through a 0.22 μm filter membrane, use the above “1.1), Hilic analysis conditions” for detection, and then the obtained The corresponding peak areas are substituted into the formula obtained in the above "1.2)" to obtain the concentrations of these 4 in the solution.
[0160] Hilic analysis chromatogram as figure 1 shown.
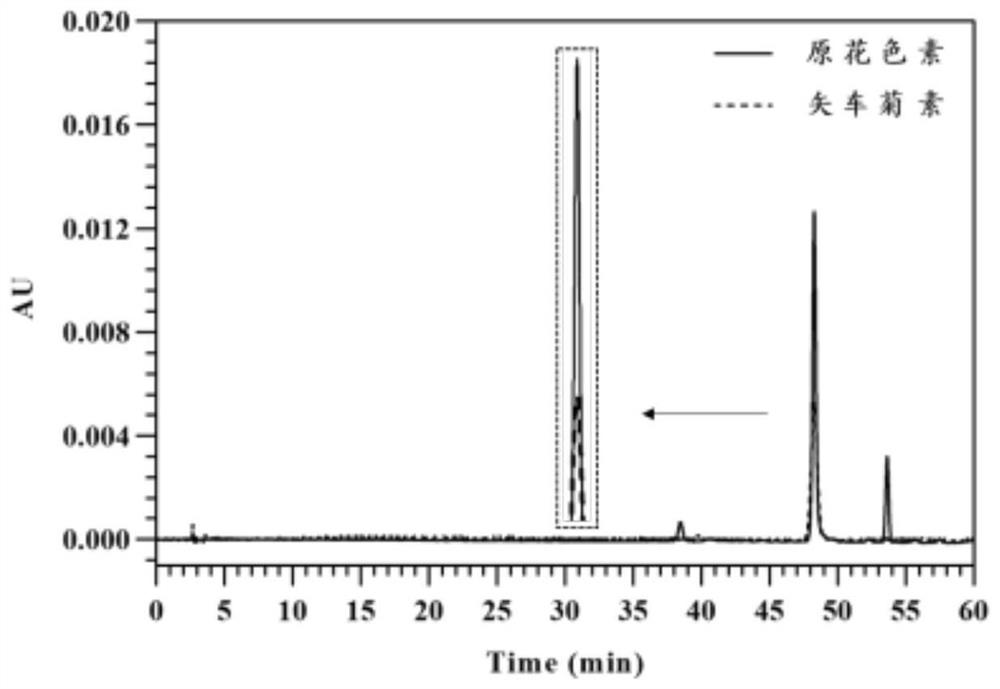
[0161] 2), Proanthocyanidin...
Embodiment 2
[0183] Embodiment 2. Cancel the "detection of the anthocyanin of the acid degradation product to be analyzed at 550 nm (as the acid degradation by-product)" in step 3 in the above embodiment 1,
[0184] Therefore, the specific calculation formula of step 4) is also correspondingly changed as follows:
[0185]
[0186] In order to compare the method of the present invention with the traditional proanthocyanidin structure analysis method, the traditional method is described in detail below.
[0187] Comparative example, traditional method:
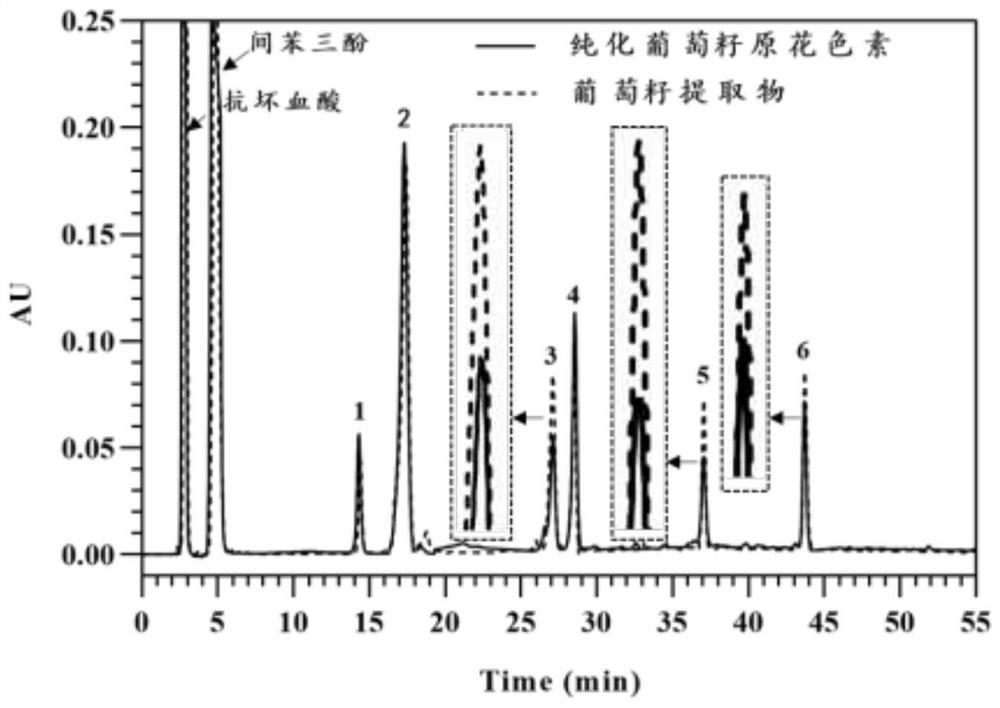
[0188] 1) Purification of proanthocyanidins: Accurately weigh 1.0 g of grape seed extract, dissolve it in 4.0 mL of 50% methanol aqueous solution, load the sample on a Sephadex LH20 column equilibrated with 50% methanol aqueous solution, and elute 5 columns with 50% methanol aqueous solution volume, collect eluent 1, then elute with 70% acetone aqueous solution for 3 column volumes, collect eluent 2, and dry eluent 2 to obtain purified g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com