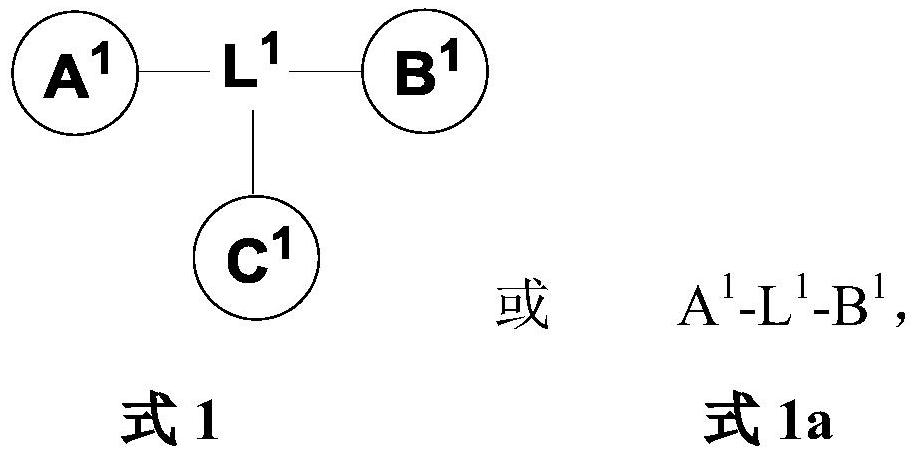
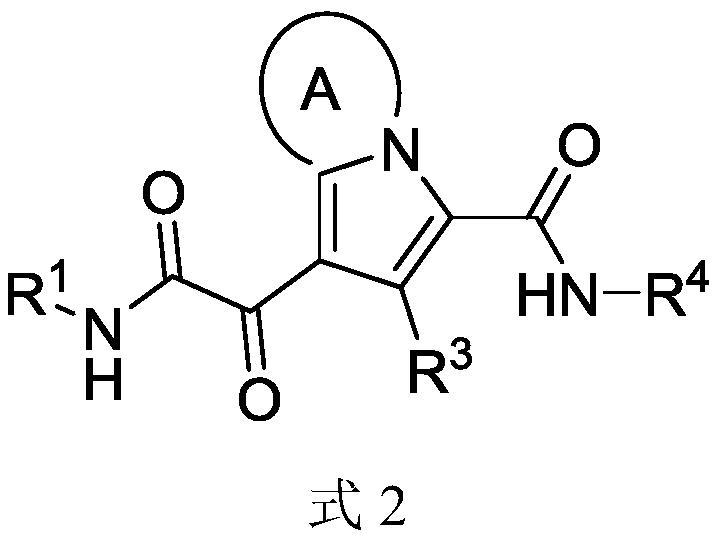
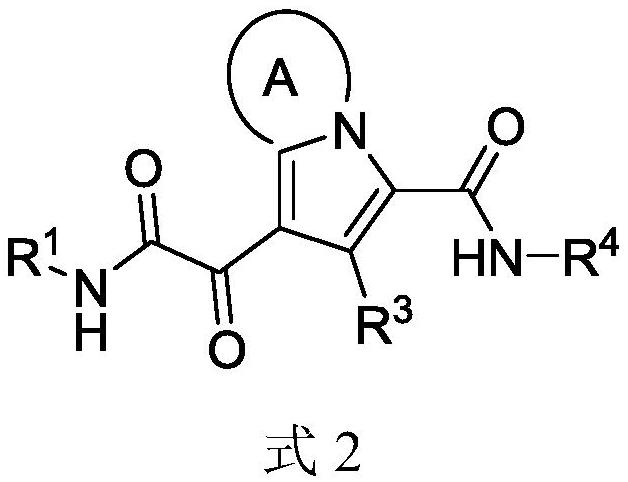
Monomer and multimeric Anti-hbv agents
A prodrug, unsaturated technology, used in antiviral agents, pharmaceutical formulations, active ingredients of boron compounds, etc., can solve the problems of affecting drug resistance, inability to provide, high remission rate, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment
[0692] Specific compounds representative of the invention were prepared according to the following examples and reaction sequences; the examples and figures depicting the reaction sequences are provided by way of illustration to aid in the understanding of the invention and should not be construed as limiting in any way the following The invention is set forth in the claims of . Compounds of the present invention may also be used as intermediates in subsequent examples to yield additional compounds of the present invention. There is no need to try to optimize the yield obtained in any reaction. Those skilled in the art will know how to increase such yields by routine changes in reaction times, temperatures, solvents and / or reagents.
[0693] Anhydrous solvents were purchased from Aldrich Chemicals, Inc. (Milwaukee, WI) and EMD Chemicals, Inc. (Gibbstown, NJ). Reagents were purchased from commercial sources. Unless otherwise stated, materials used in the examples were obtain...
Embodiment 1
[0695]
[0696] Scenario 19
[0697] Reagents and conditions: a) i) benzyl 3-oxobutyrate, AcOH, iPrOH, piperidine, 50°C, 1h;
[0698] ii) pyridine-2-carboxamidine hydrochloride, 125°C, 3 days; b) i) NBS, AcOH, DCE, 50°C, 30 min; ii) morpholine, Et 3 N, DMF, RT, 18h; c)H 2 , Pd / C, EtOH, RT, 1h; d) NaI, Na 2 CO 3 , oxone, MeOH / H 2 O, 1 h, RT; e) CbzCl, KHMDS, THF, -78°C to RT, 15 h; f) ethynylcyclopropane, PdCl 2 (PPh 3 ) 2 , CuI, Et 3 N, DMF, RT, 4h; g) BCl 3 , DCM, -78°C, 3h.
[0699] Benzyl 4-(2-chloro-4-fluorophenyl)-6-methyl-2-(pyridin-2-yl)-1,4-dihydropyrimidine-5-carboxylate (2)
[0700] To a suspension of 2-chloro-4-fluorobenzaldehyde (6 g, 37.8 mmol), piperidine (3 mL), acetic acid (1 mL) in dry isopropanol (60 mL) was added benzyl 3-oxobutyrate (10.9 g, 56.8 mmol). The mixture was heated at 50°C for 1 hour, then pyridine-2-carboxamidine hydrochloride (5.96 g, 37.8 mmol) was added. The resulting solution was kept at 125°C for 3 days and then concentrat...
Embodiment 2
[0976] Cytotoxicity assay
[0977] As previously described (see Schinazi R.F., Sommadossi J.-P., Saalmann V., Cannon D.L., Xie M.-Y., Hart G.C., Smith G.A. & Hahn E.F. Antimicrob. Agents Chemother. 1990, 34, 1061-67) , the toxicity of compounds was assessed in Vero, human PBM, CEM (human lymphoblastoid), MT-2 and HepG2 cells. Cycloheximide was included as a positive cytotoxicity control, and untreated solvent-exposed cells were included as a negative control. Cytotoxicity ICs were obtained from concentration-response curves using the previously described median efficient method 50 (See Chou T.-C. & Talalay P. Adv. Enzyme Regul. 1984, 22, 27-55; Belen'kii M.S. & Schinazi R.F. Antiviral Res. 1994, 25, 1-11). The results are shown in Table 1 below:
[0978] Table 1
[0979] Cytotoxicity, CC 50 , μM (% inhibition)
[0980]
[0981]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap