Crystal form of hydronidone, preparation method and application thereof
A technology of oxynidone and crystal form, applied in the field of medicinal chemistry, can solve the problems of low solubility and dissolution rate of oxynidone, unsatisfactory bioavailability, poor stability, etc., and achieve good application value and good reproducibility , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Embodiment 1 (preparation of amorphous Oxynione A)
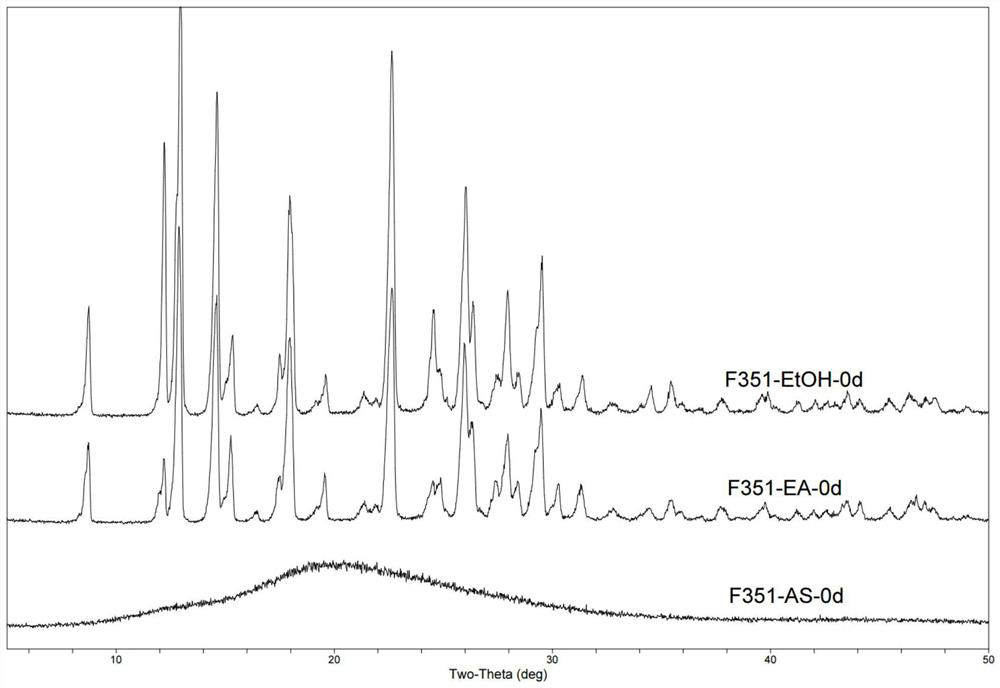
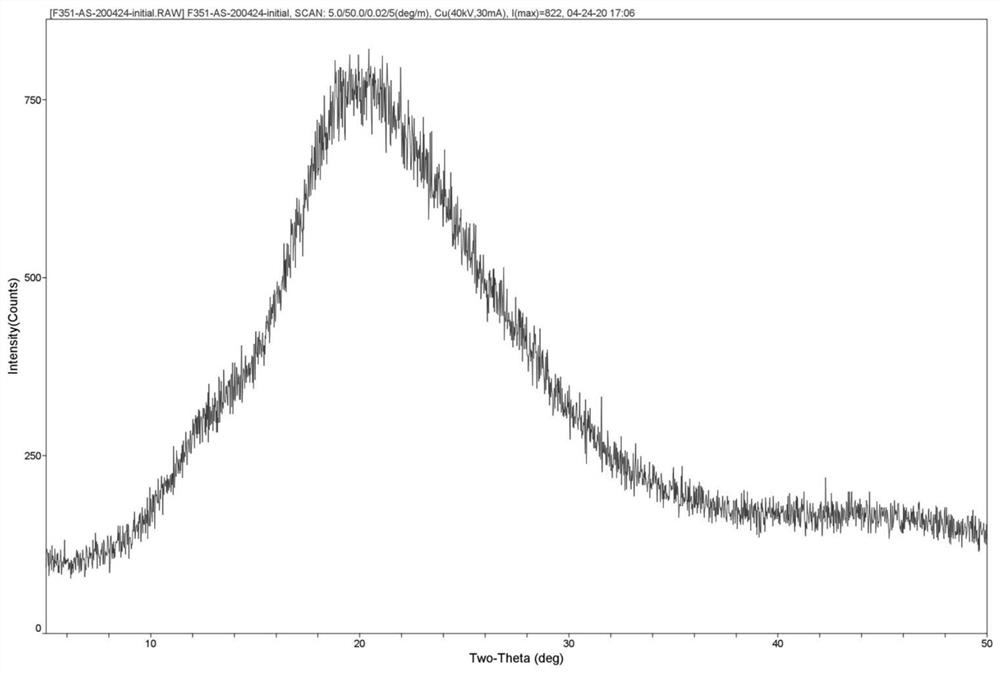
[0100] About 11 g of F351 was placed in a 50 mL ceramic crucible. The crucible is then placed in the muffle furnace. Set the temperature of the muffle furnace at 190°C, and heat it within about 40 minutes until the contents are completely melted. In addition, prepare an iron pan in advance, and place the iron pan in an ice-water bath to keep the temperature constant. After the F351 in the crucible was completely melted, it was poured into the iron pan to obtain 10.5 g of a light yellow transparent lumpy solid, which was designated as Amorphous A. It was used directly in subsequent experiments.
Embodiment 2
[0101] Example 2 (recrystallization in ethyl acetate to obtain crystal form B1)
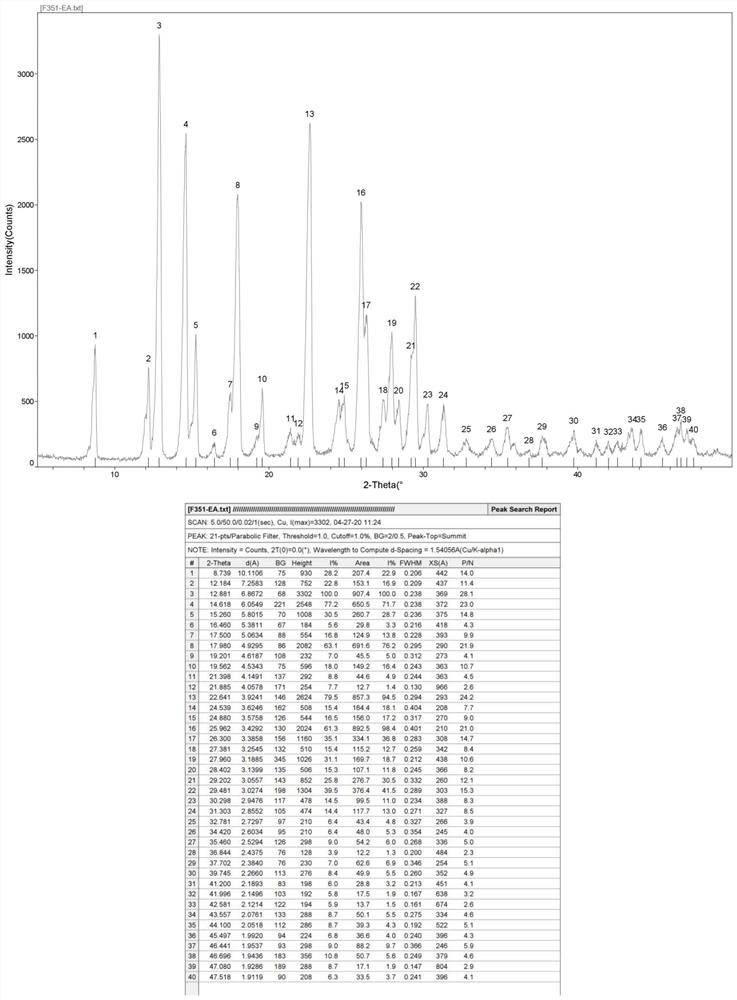
[0102] 10g of F351 was placed in a 500ml single-necked bottle, and 300ml of ethyl acetate was added thereto. The mixture was heated to reflux to obtain a suspension, during which stirring was continued for 30 min. The temperature of the obtained solution was lowered to room temperature (the cooling rate was about 2° C. / min), during which solids were gradually precipitated. It was filtered and vacuum-dried to obtain 8 g of white powder, which was designated as Form B1 and used directly in subsequent experiments.
Embodiment 3
[0103] Example 3 (recrystallization in ethanol to obtain crystal form B2)
[0104] Put 10g of F351 in a 500ml single-necked bottle, and add 50ml of ethanol to it. The mixture was heated to reflux and the solids were all dissolved until a clear solution was obtained, stirring during this and continued for 30 min after a clear solution was obtained. The temperature of the obtained solution was lowered to room temperature (the cooling rate was about 2° C. / min), during which solids were gradually precipitated. It was filtered and vacuum-dried to obtain 7.7 g of white powder, which was designated as Form B2, which was directly used in subsequent experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com