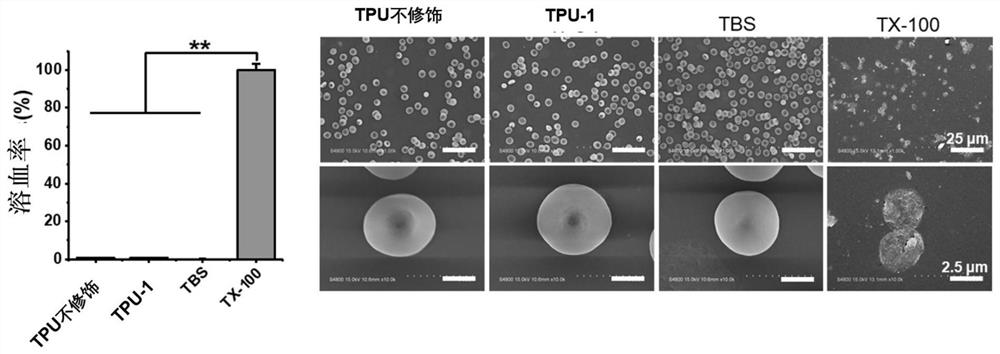
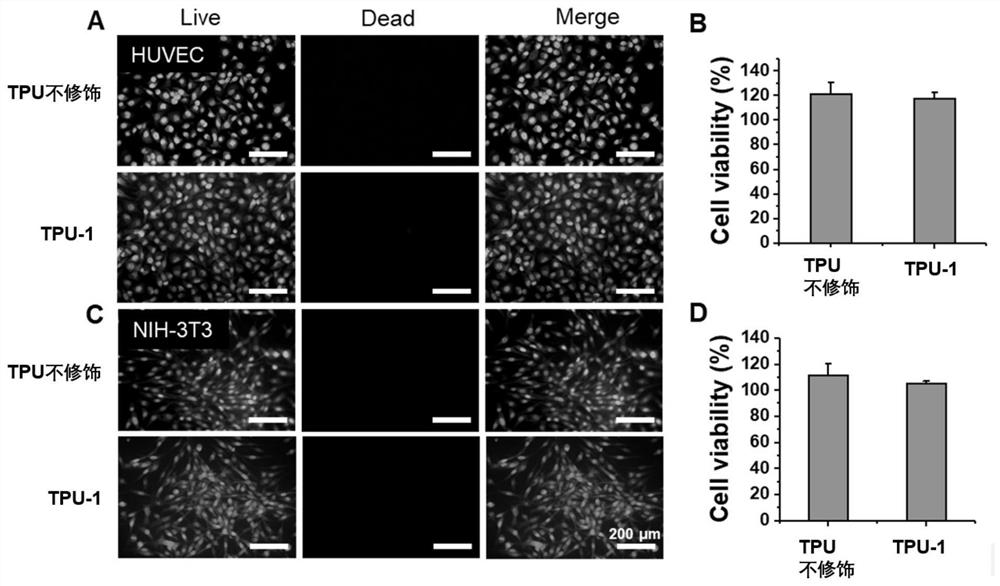
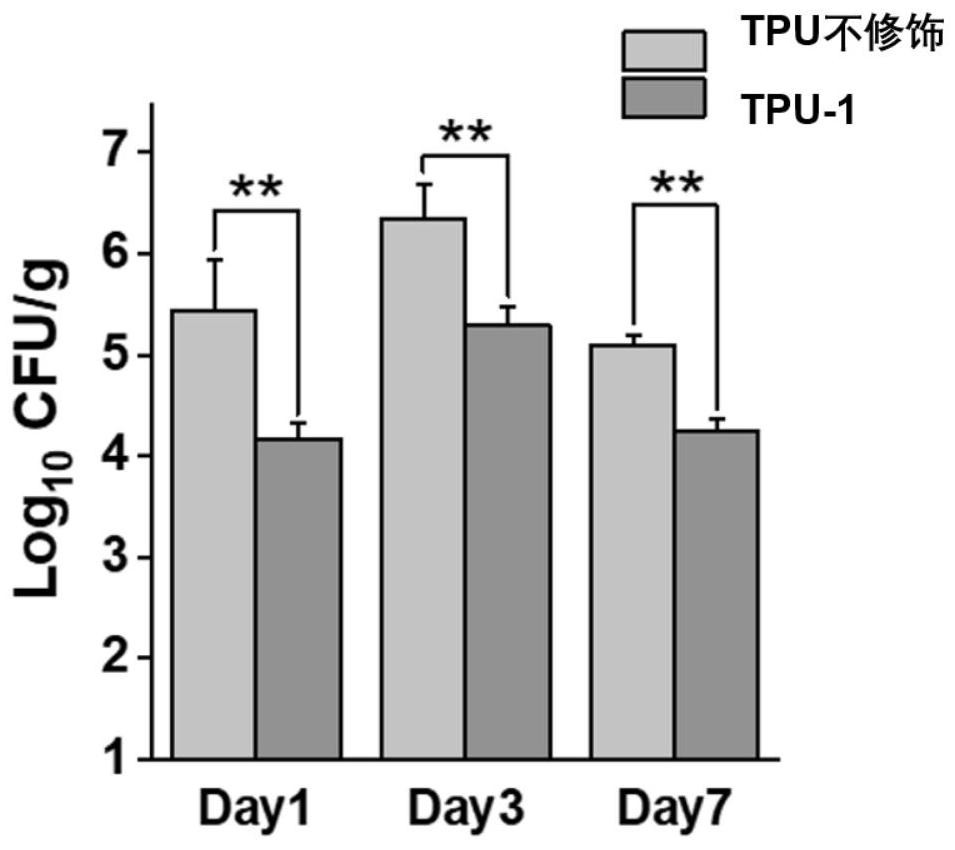
Method for improving antibacterial activity and biocompatibility of surface of base material
A biocompatible, substrate surface technology, applied in coatings, medical science, absorbent pads, etc., can solve problems such as threatening the health of patients, easily causing microbial infections, and economic losses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0178] Example 1: Lithium hexamethyldisilazide (LiHMDS) initiated N-ε-tert-butoxycarbonyl-DL-lysine-N-carboxyl anhydride and L-glutamic acid-5-benzyl ester Preparation of Random α-Amino Acid Copolymers with -N-Carboxyl Intracyclic Anhydrides
[0179] Lithium hexamethyldisilazide (33.4mg, 0.2mmol) was accurately weighed and prepared into a 0.1M solution with tetrahydrofuran (2mL) for later use. Weigh N-ε-tert-butoxycarbonyl-DL-lysine-N-carboxyl ring anhydride and L-glutamic acid-5-benzyl-N-carboxyl ring anhydride, and use tetrahydrofuran as a solvent. Take 1.8mL N-ε-tert-butoxycarbonyl-DL-lysine-N-carboxyl anhydride (0.2M) and 0.2mL-glutamic acid-5-benzyl ester-N-carboxyl anhydride (0.2M ) after mixing, add a magnet for stirring (taking monomer ratio x:y=9:1 as an example). Into a stirred reaction vial, add 0.8 mL of a 0.1 M lithium hexamethyldisilazide salt solution. The mixture was reacted with stirring at room temperature for 5 minutes in a glove box. After the polymer...
Embodiment 2
[0181] Example 2: Lithium hexamethyldisilazide (LiHMDS) triggers N-ε-tert-butoxycarbonyl-DL-lysine-N-carboxyl anhydride and DL-norleucine-N-carboxy Preparation of Random α-Amino Acid Copolymers with Intracyclic Anhydrides
[0182] The experimental method is the same as in Example 1, the difference is that DL-norleucine-N-carboxyl anhydride is replaced by L-glutamic acid-5-benzyl ester-N-carboxyl anhydride; The 0.1 M lithium hexamethyldisilazide salt solution was replaced by 0.8 mL of 0.1 M lithium hexamethyldisilazide salt solution. The obtained polymer had a molecular weight Mn=7500 g / mol and a molecular weight distribution Mw / Mn=1.19. The subunit ratio of the polymer was obtained by characterization by H NMR spectroscopy, and x:y=6.12:4 in the final polymer.
Embodiment 3
[0183] Example 3: Lithium hexamethyldisilazide (LiHMDS) triggers N-ε-tert-butoxycarbonyl-DL-lysine-N-carboxyl anhydride and DL-norvaline-N-carboxyl Preparation of Random α-Amino Acid Copolymers with Intracyclic Anhydrides
[0184] The experimental method is the same as in Example 1, the difference is that DL-norvaline-N-carboxyl anhydride is replaced by L-glutamic acid-5-benzyl ester-N-carboxyl anhydride; The 0.1 M lithium hexamethyldisilazide salt solution was replaced by 0.8 mL of 0.1 M lithium hexamethyldisilazide salt solution. The obtained polymer had a molecular weight Mn=7100 g / mol and a molecular weight distribution Mw / Mn=1.19. The subunit ratio of the polymer was obtained by H NMR spectrum characterization, and x:y=6:4 in the final polymer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
| surface roughness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com