Application of curcumin analogue CUR5g as novel autophagy inhibitor
A technology of curcumin analogs and autophagy inhibitors, applied in the application field of curcumin derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
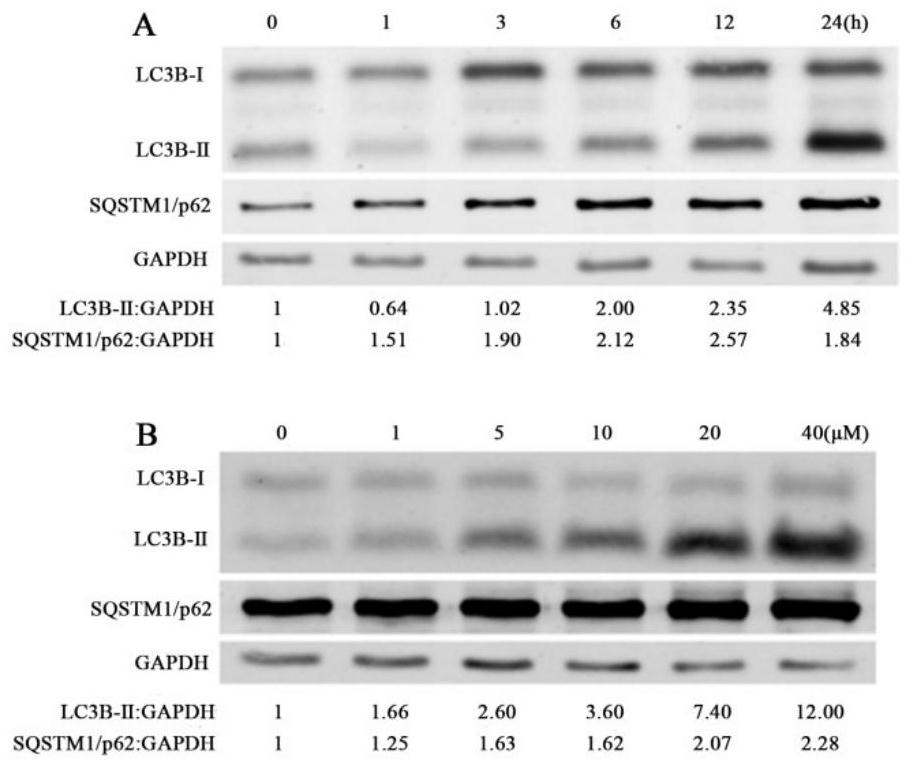
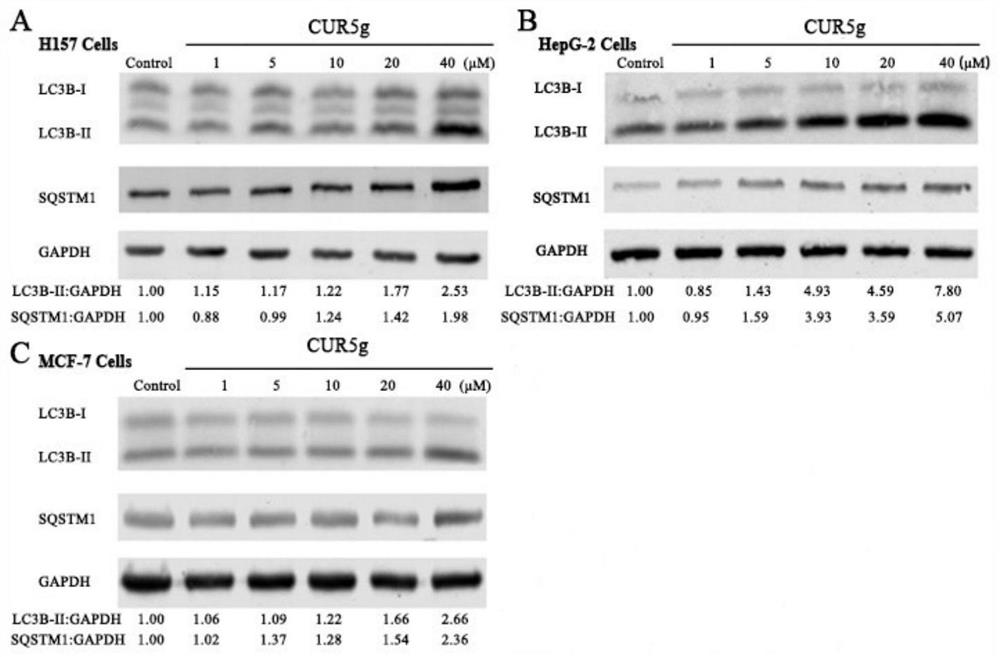
[0030] The following demonstrates the role of CUR5g as an autophagy inhibitor in combination with specific pharmacological experiments and results examples of the curcumin analogue CUR5g.
[0031] Preparation of A549 cells: A549 cells were cultured by conventional methods, and A549 cells in good growth state and in logarithmic growth phase were selected for use.
[0032] Combining the methods of cell biology and molecular biology, the following experiments were carried out to observe the effect of CUR5g on autophagy inhibition.
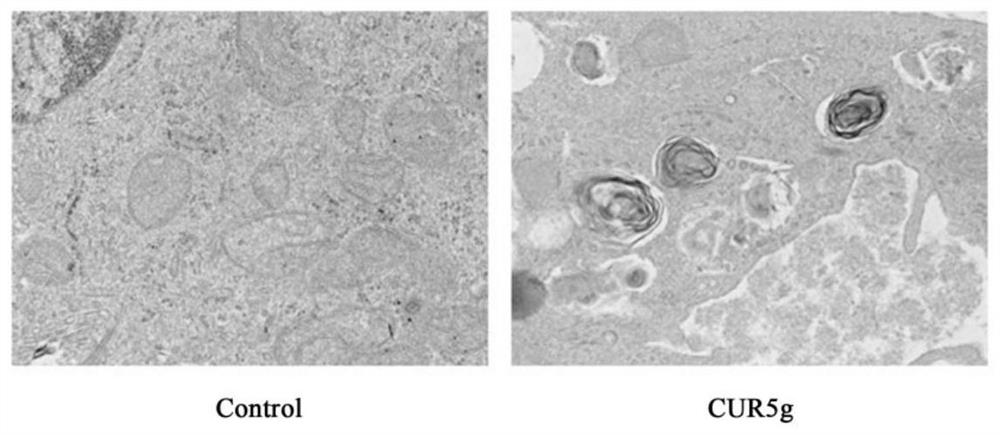
[0033] EXAMPLES Experiment 1. Detecting the effect of CUR5g on autophagy of A549 cells by transmission electron microscopy:
[0034] A549 cells were seeded into 100mm culture dishes. Set up the control group: add dimethyl sulfoxide (DMSO) equal to the volume of the drug for 24 hours; set up the experimental group: add 10 μM CUR5g for 24 hours. Rinse the cells twice with pre-cooled 1×PBS, add 5ml of pre-cooled 2.5% glutaraldehyde to the culture dish,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com