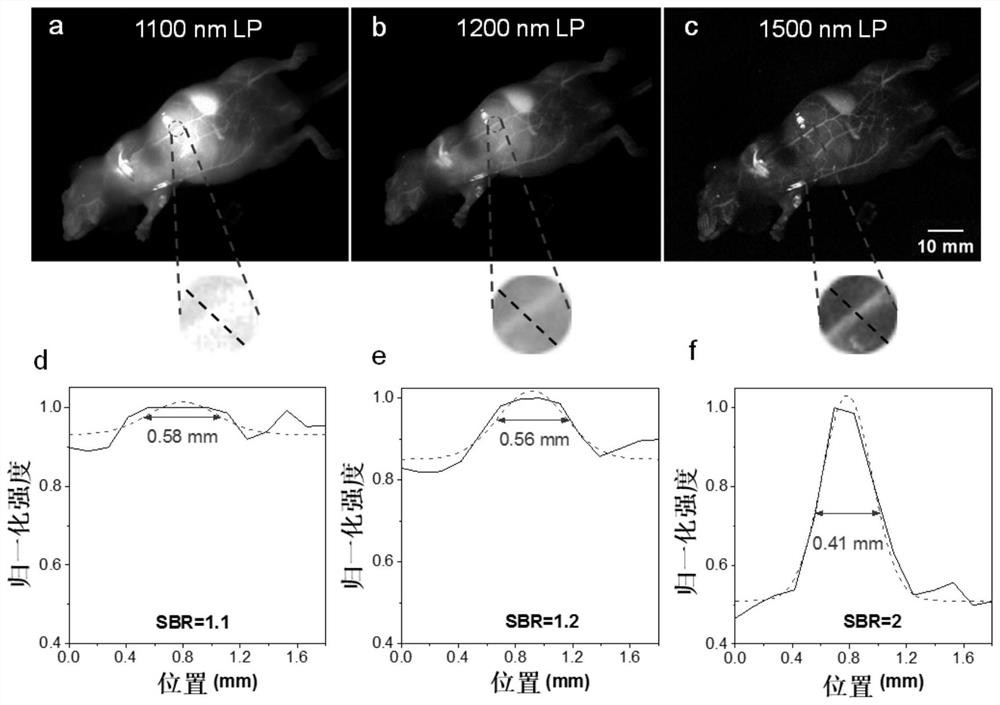
Near-infrared two-region aggregation-induced emission fluorescent compound as well as preparation method and application thereof
A fluorescent compound and selected technology, applied in the field of biomedical fluorescence imaging applications, can solve problems such as luminescence quenching and difficulty in developing fluorescent aggregate nanoparticles
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
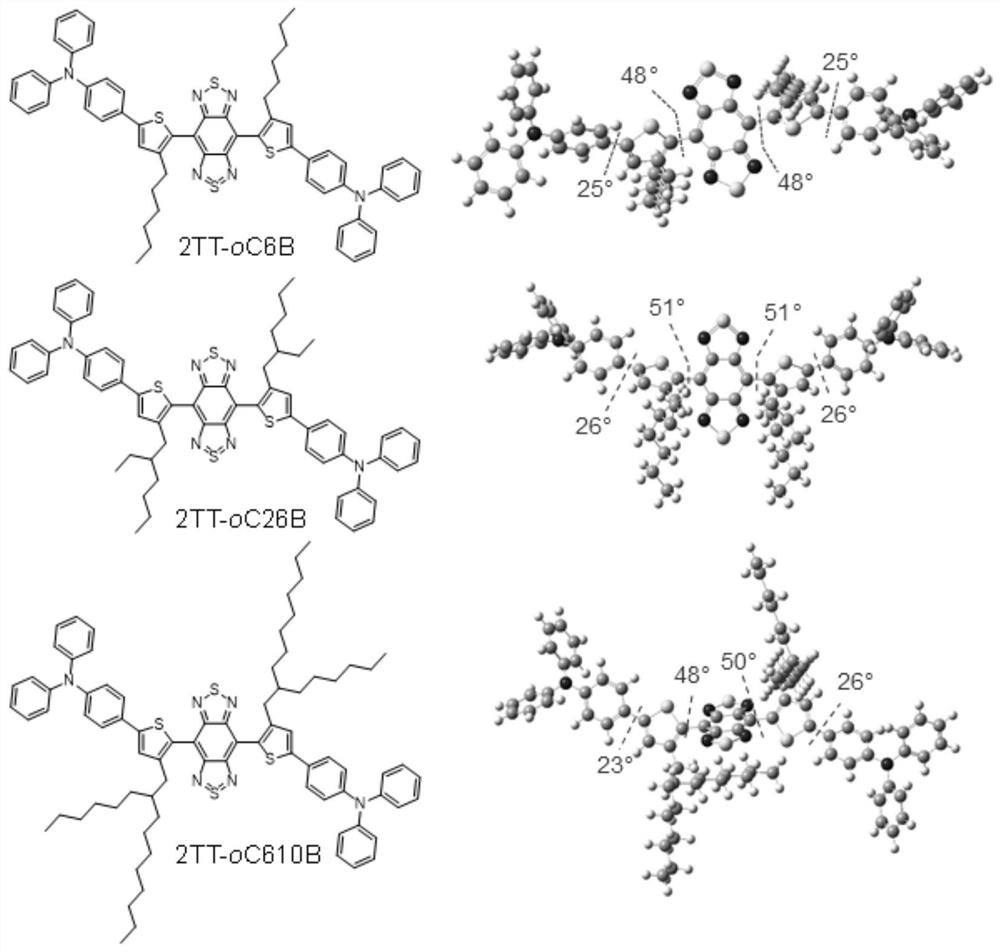
[0040] According to some embodiments of the present invention, the fluorescent compound has a structure represented by general formula (I):
[0041]
[0042] According to some embodiments of the present invention, R 1 and R 2 It is a hydrophobic steric hindrance group, which is used to ensure the deformation of the molecular conjugated skeleton.
[0043] According to a preferred embodiment of the present invention, R 1 and R 2 The same or different, and each independently selected from C1-C30 alkyl, C3-C30 cycloalkyl, C2-C30 heterocycloalkyl, C6-C30 aryl, C4-C30 heteroaryl, C1-C30 alkoxy and -R 10 N(R 11 )R 12 .
[0044] According to a preferred embodiment of the present invention, R 1 and R 2 The same or different, and each independently selected from C1-C30 straight chain alkyl, C3-C30 branched chain alkyl, C3-C30 cycloalkyl, C2-C30 heterocycloalkyl, C6-C30 aryl, C7-C30 Aralkyl, C7-C30 alkaryl, C4-C30 heteroaryl, C1-C30 alkoxy and -R 10 N(R 11 )R 12 .
[004...
Embodiment 1
[0180] Preparation of compound 2TT-oC26B
[0181]
[0182] In a 10ml test tube, add organotin compound (0.7g, about 1mmol) shown in formula (a1), dibromo-benzobisthiadiazole (87mg, about 0.25mmol) shown in formula (b), Pd 2 (dba) 3 (22mg, about 0.025mmol), P(o-tol) 3 (66 mg, about 0.21 mmol) and degassed dry toluene (1.5 mL), and seal with a cap to give a mixture. The mixture was stirred and heated to 130° C. for 48 h under a nitrogen atmosphere to obtain a crude product. After cooling the crude product was quenched with KF and extracted with DCM. The combined organic phases were washed with Na 2 SO 4 After drying and removing the solvent, the product was purified by a silica gel column to obtain a dark green solid (yield 35%). 1 H NMR (400MHz, CDCl 3 ),δ(ppm)=7.59-7.56(4H,m),7.31-7.29(10H,m),7.16-7.04(16H,m),2.59-2.57(4H,m),1.63(2H,m), 1.15-1.10(16H,m),0.73(12H,m). 13 C NMR (100MHz, CDCl 3), δ (ppm): 152.53, 146.81, 128.86, 128.70, 127.54, 126.12, 124.99, 123.94,...
Embodiment 2
[0184] Preparation of compound 2TT-oC610B
[0185]
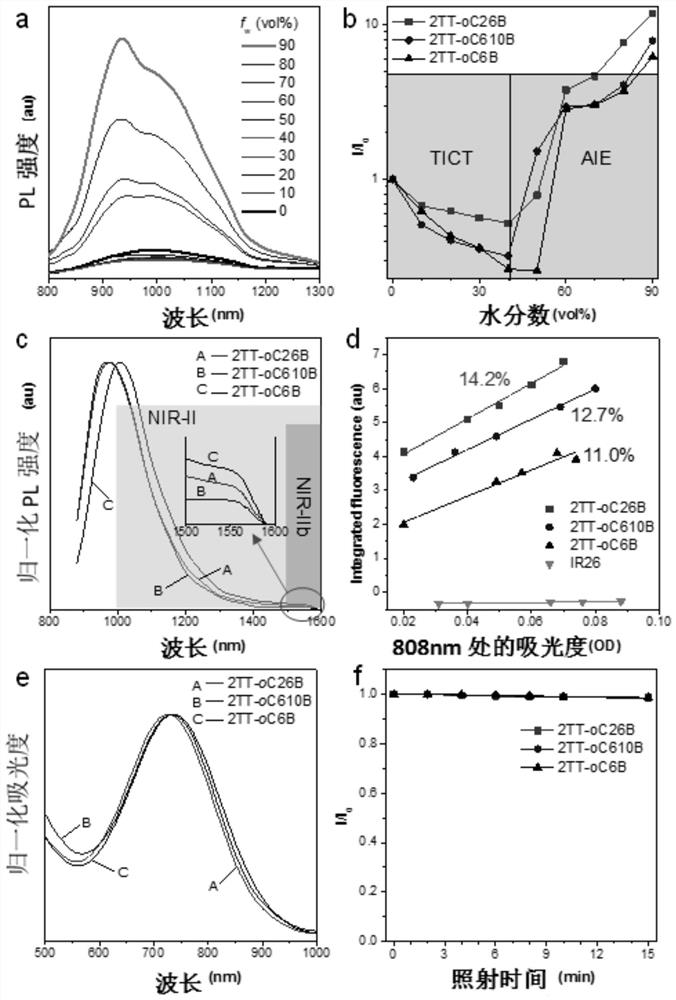
[0186] The preparation method was the same as in Example 1, except that the organotin compound represented by formula (a1) was replaced by the organotin compound represented by formula (a2) (0.84 g, about 1 mmol) to obtain a dark green solid (50% yield). 1 H NMR (400MHz, CDCl 3 ), δ(ppm)=7.49-7.47(4H,m),7.33-7.29(10H,m),7.18-7.01(16H,m),2.79(4H,m),1.83(2H,m),1.23( 48H,m), 0.83(12H,m). 13 C NMR (100MHz, CDCl 3 ),δ(ppm):153.19,152.54,146.92,146.78,145.98,144.02,132.38,128.86,128.70,128.17,127.59,126.09,124.96,123.99,123.29,122.87,122.48,120.77,115.77,38.62,32.60,31.18 , 29.24, 28.86, 25.78, 22.07, 13.51. MS:m / z:[M]+calcd for C 82 h 96 N 6 S 4 :1292.6579,found:1292.6595. The fluorescence quantum yield was 11%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com