Synthesis of tolvaptan degradation derivative
A technology of tolvaptan and its derivatives, which is applied in the field of synthesis of tolvaptan degraded derivatives, achieving the effects of simple experimental operation, reasonable route design, and cheap raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
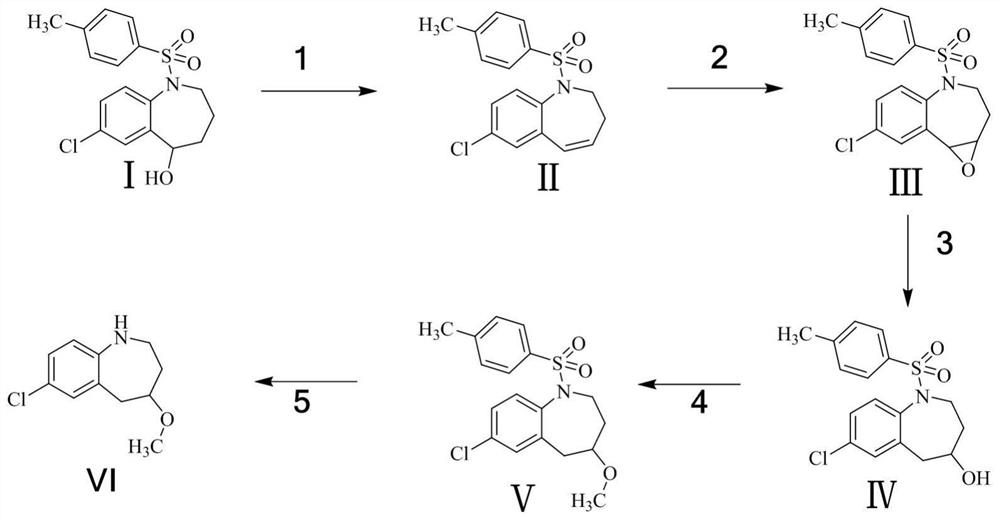
[0031] Such as figure 1 Shown, a kind of synthetic method of tolvaptan degradation derivative comprises the following steps:
[0032] Step (1): Preparation of Compound II
[0033] Take 3.0 g of raw material I and dissolve in 30 mL of toluene, then add 1.67 g of concentrated sulfuric acid, react at 110 ° C, and monitor the completion of the reaction by TLC. The reaction solution was washed with saturated aqueous sodium bicarbonate solution, then directly concentrated and evaporated to dryness, and purified by column chromatography to obtain 2.5 g of compound II with a yield of 87.8%.
[0034]
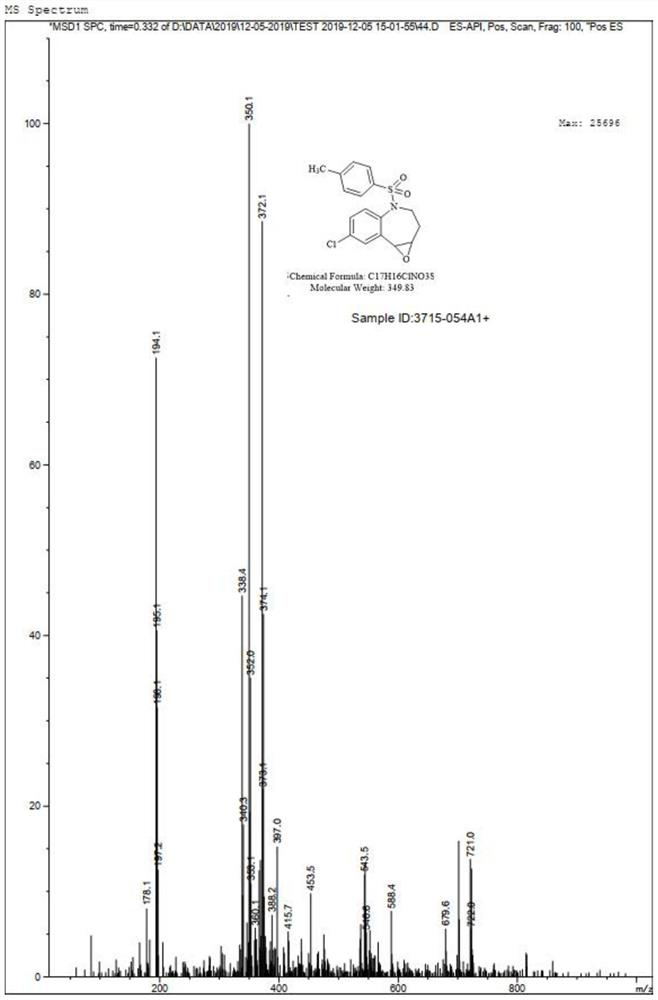
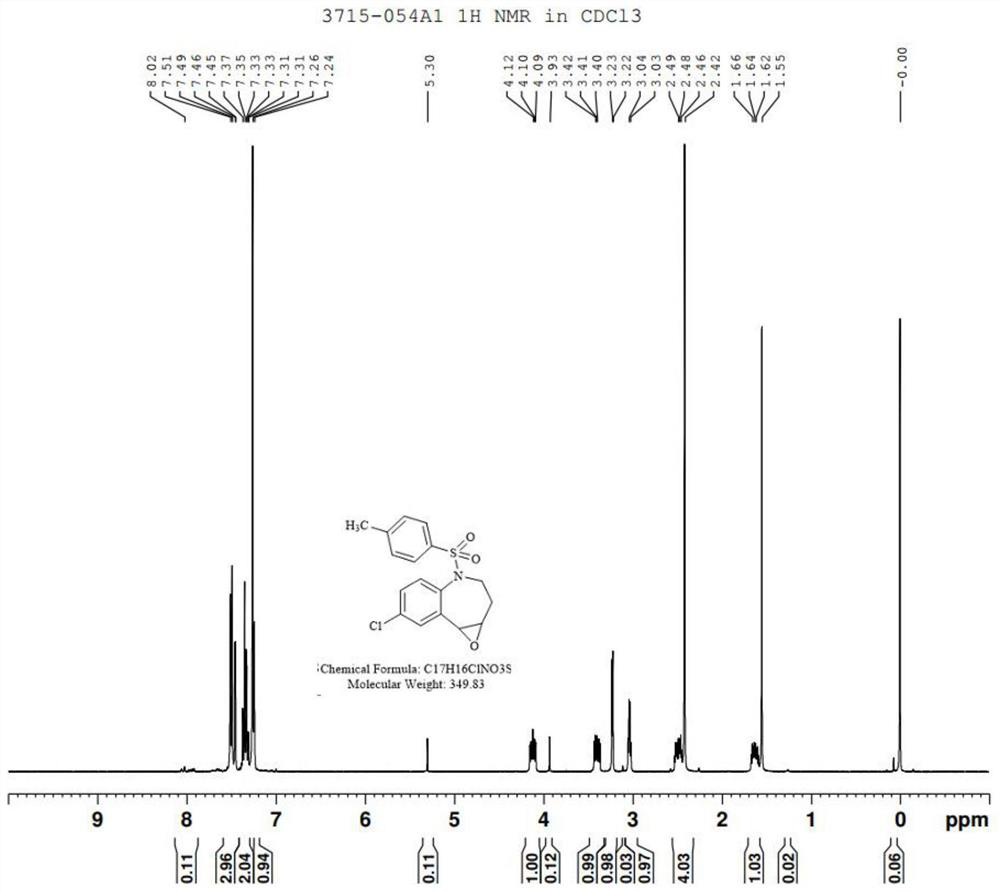
[0035] Step (2): Preparation of Compound III
[0036] Take 2.5g of compound II and dissolve it in 50mL of methanol, add 1.04g of hydrogen peroxide, after a period of reaction, TLC shows that the reaction is complete. Sodium sulfite solution was added under ice-cooling to quench the remaining oxidant, methanol was concentrated, the aqueous phase was extracted with ethyl acetate, and...
Embodiment 2
[0048] Such as figure 1 Shown, a kind of synthetic method of tolvaptan degradation derivative comprises the following steps:
[0049] Step (1): Preparation of Compound II
[0050] Take 9.0 g of raw material I and dissolve it in 90 mL of toluene, then add 5 g of concentrated sulfuric acid, react at 110 ° C, and monitor the completion of the reaction by TLC. The reaction solution was washed with saturated aqueous sodium bicarbonate solution, then directly concentrated and evaporated to dryness, and purified by column chromatography to obtain 7.47 g of compound II with a yield of 87.5%.
[0051]
[0052] Step (2): Preparation of Compound III
[0053] Take 7.3g of compound II and dissolve it in 120mL of methanol, add 2.5g of hydrogen peroxide, after a period of reaction, TLC shows that the reaction is complete. Add sodium sulfite solution under ice bath to quench the remaining oxidant, concentrate to remove methanol, extract the aqueous phase with ethyl acetate, and concentr...
Embodiment 3
[0065] Such as figure 1 Shown, a kind of synthetic method of tolvaptan degradation derivative comprises the following steps:
[0066] Step (1): Preparation of Compound II
[0067] Take 45.0g of raw material I and dissolve in 270mL of toluene, then add 15.03g of concentrated sulfuric acid, react at 110°C, and monitor the completion of the reaction by TLC. The reaction solution was washed with saturated aqueous sodium bicarbonate solution, then directly concentrated and evaporated to dryness, and purified by column chromatography to obtain 37.23 g of compound II with a yield of 87.2%;
[0068]
[0069] Step (2): Preparation of Compound III
[0070] Take 37.0g of Compound II and dissolve it in 720mL of methanol, add 14.98g of hydrogen peroxide, after a period of reaction, TLC shows that the reaction is complete. Sodium sulfite solution was added under an ice bath to quench the remaining oxidant, methanol was concentrated, the aqueous phase was extracted with ethyl acetate, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com