Synthesis method of nimustine hydrochloride
A technology of nimustine hydrochloride and a synthesis method, which is applied in the field of drug synthesis, can solve the problems of hidden danger of operator's personal safety, inability to accurately calculate yield, and high operational risk, and achieves avoiding side reactions, reducing reaction toxicity, The effect of improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 A kind of synthetic method of nimustine hydrochloride A1
[0027] The present embodiment provides a kind of synthetic method of nimustine hydrochloride A1, and its synthetic method is as follows:
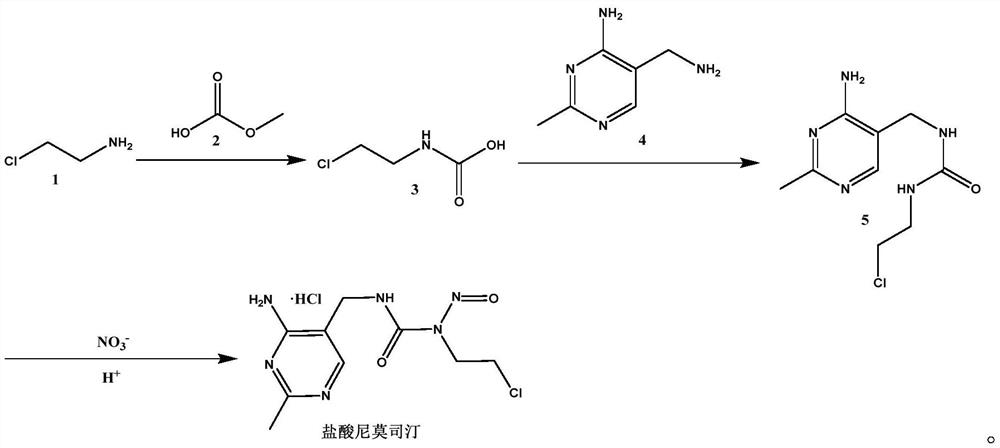
[0028] 1) Weigh 79.53g of 2-chloroethylamine and 76.05g of methyl bicarbonate into 300ml of methanol, mix and stir under the protection of nitrogen, after stirring evenly, raise the temperature to 80°C and keep it warm for 1h, take a small amount of reaction solution after 1h , injected into the liquid chromatograph after dilution and carried out routine condition analysis, monitoring the progress of the reaction, the molecular weight appearing in the obtained spectrogram is 122.54 (M - ), and the absorption peak of 2-chloroethylamine disappeared, that is, the end of the reaction was reached. After the reaction, the reaction solution was down to room temperature, concentrated under reduced pressure to dryness, and obtained 101.3g of compound (3), Yield: 82%;
...
Embodiment 2~5
[0035] Embodiment 2~5 a kind of synthetic method of nimustine hydrochloride A2~A5
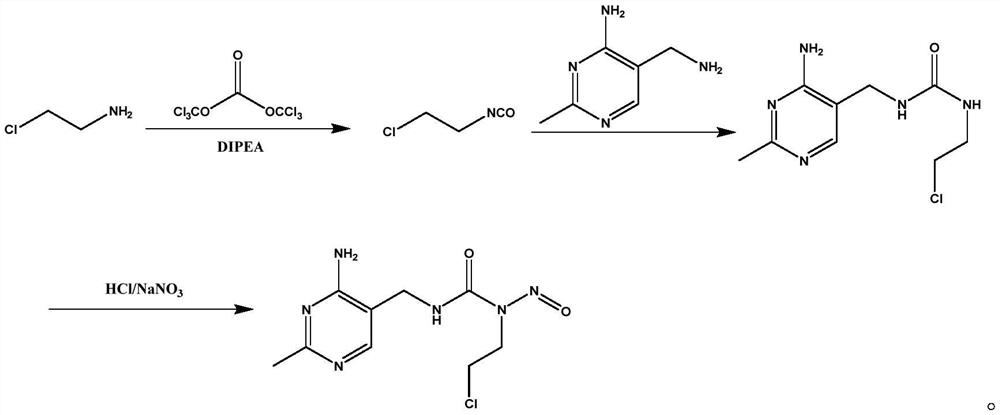
[0036] The synthetic method of the nimustine hydrochloride A2~A5 that embodiment 2~5 provides, their synthetic method and the synthetic method of the nimustine hydrochloride provided in the embodiment 1 are basically the same, the difference is only different in some process parameters, The specific process parameters are shown in Table 1.
[0037] Table 1: Process parameter list of nimustine hydrochloride A2~A5
[0038]
[0039]
[0040] *: The concentration of dilute nitric acid is 68%; the concentration of hydrochloric acid is 36%.
[0041] Other parameters are all the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com