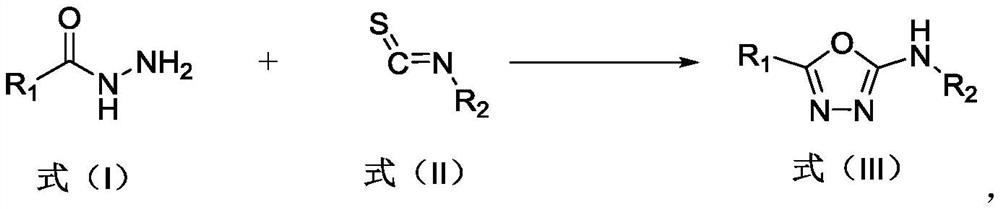
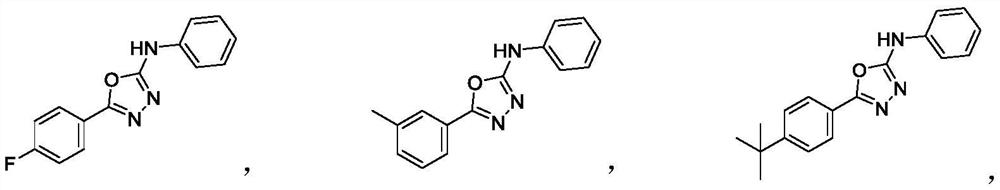
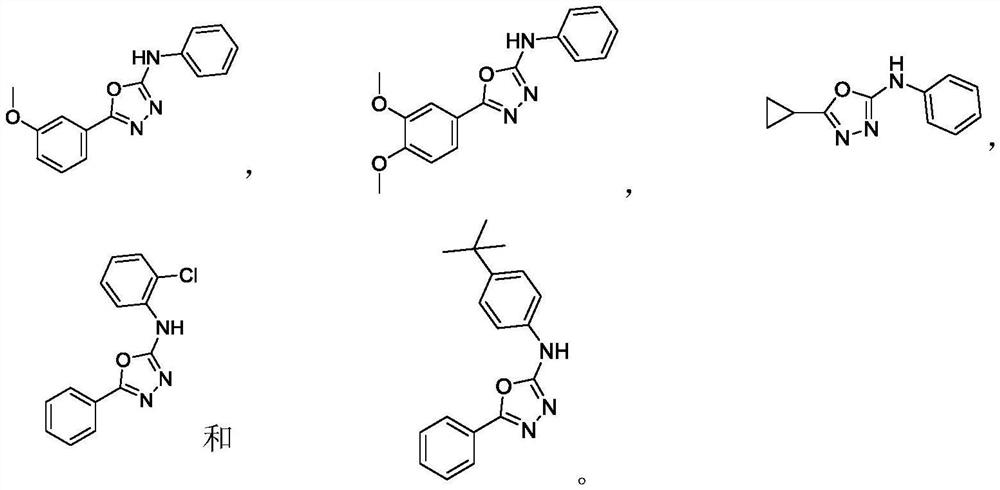
Preparation method of 2-amino-1, 3, 4-oxadiazole compound and prepared compound
A technology of oxadiazoles and compounds, applied in the field of medicinal chemistry, can solve problems such as harsh conditions, expensive catalysts, unfavorable industrial production, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 N, the preparation of 5-diphenyl-1,3,4-oxadiazol-2-amine
[0030]Add benzohydrazide (0.20 g, 1.46 mmol), phenylisothiocyanate (0.20 g, 1.5 mmol), dimethyl sulfoxide (DMSO) 3 mL into a 50 mL eggplant-shaped flask, stir at room temperature for 30 min, add KHSO 4 (1.19 g, 8.75 mmol), the reaction was continued at room temperature for 6 h. After the reaction was completed, 30 ml of water was added, filtered, and the solid was dried and purified by column chromatography to obtain 0.29 g of a light red solid with a yield of 84%. m.p.207-209°C; 1 H NMR (600 MHz, DMSO-d 6 )(δ,ppm):10.68(s,1H),7.98–7.82(m,2H),7.69–7.50(m,5H), 7.37(dd,J=8.2,7.6Hz,2H),7.02(t, J=7.3Hz,1H); 13 CNMR (150MHz, DMSO-d 6 )(δ,ppm):160.38,158.19,139.10,131.44,129.82,129.57,126.00,124.33, 122.36,117.53. HRMS(ESI):m / z[M+Na]+Calcd for C14H11N3O:237.0902; .
Embodiment 2
[0031] Example 2 N, the preparation of 5-diphenyl-1,3,4-oxadiazol-2-amine
[0032] Add benzohydrazide (0.20 g, 1.46 mmol), phenylisothiocyanate (0.20 g, 1.5 mmol), dimethyl sulfoxide (DMSO) 3 mL into a 50 mL eggplant-shaped flask, stir at room temperature for 30 min, add KHSO 4 (1.79 g, 13.14 mmol), the reaction was continued at room temperature for 2 h. After the reaction was completed, 30 ml of water was added, filtered, and the solid was dried and purified by column chromatography to obtain 0.22 g of a light red solid with a yield of 63%.
Embodiment 3
[0033] Example 3 N, the preparation of 5-diphenyl-1,3,4-oxadiazol-2-amine
[0034] Add benzohydrazide (0.20 g, 1.46 mmol), phenylisothiocyanate (0.20 g, 1.5 mmol), dimethyl sulfoxide (DMSO) 3 mL into a 50 mL eggplant-shaped flask, stir at room temperature for 30 min, add KHSO 4 (0.59 g, 4.38 mmol), the reaction was continued at room temperature for 12 h. After the reaction was completed, 30 ml of water was added, filtered, and the solid was dried and purified by column chromatography to obtain 0.24 g of a light red solid with a yield of 70%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com