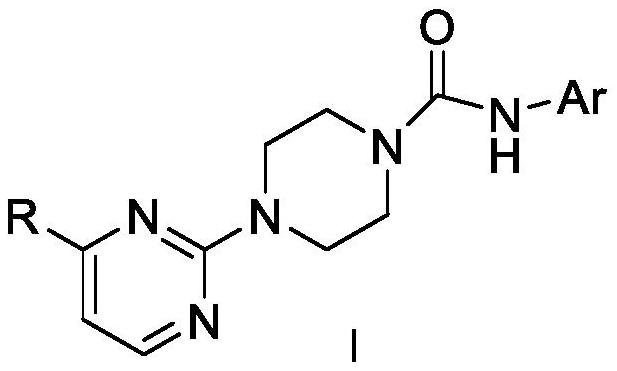
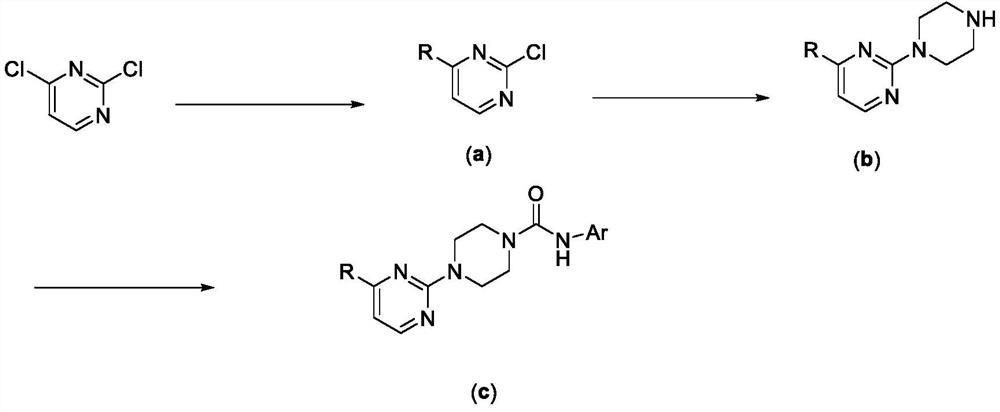
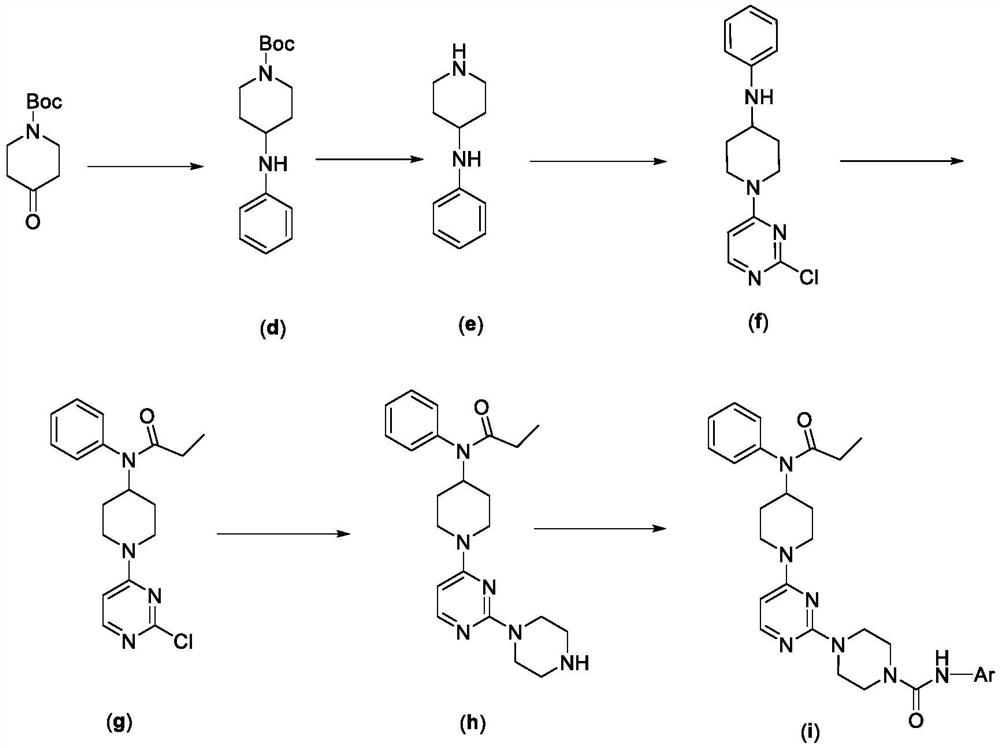
Pyrimidyl piperazine urea TRPV1 antagonistic/MOR agonistic double-target compound as well as preparation method and application thereof
A technology of pyrimidinylpiperazine urea and pyrimidinylpiperazine, which is applied in the field of medicinal chemistry, can solve problems such as loss of thermal perception and hinder development, and achieve the effects of blocking pain transmission, reducing side effects, and having good application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preparation of N-(4-bromophenyl)-4-(4-(pyrrolidin-1-yl)pyrimidin-2-yl)piperazine-1-carboxamide (compound 1) according to synthetic route A:
[0046] (1) At 0°C, triethylamine (3.84mL) was added dropwise to 2,4-dichloropyrimidine (2.05g, 13.8mmol) in dichloromethane solution (30mL) and stirred, then pyrrolidine (0.985g , 13.8mmol) of dichloromethane (20mL) was added dropwise to the above reaction system, heated to room temperature, reacted for 4h, and detected by TLC. After the reaction was completed, it was washed with saturated brine (30mL×3), and the organic phase was washed with anhydrous MgSO 4 Dry, filter, concentrate under reduced pressure, and separate and purify by column chromatography (PE:EA=50:1) to obtain 2-chloro-4-(pyrrolidin-1-yl)pyrimidine (compound a).
[0047] (2) Weigh the 2-chloro-4-(pyrrolidin-1-yl)pyrimidine (1.50g, 8.194mmol) prepared in step (1), anhydrous piperazine (0.705g, 8.194mmol), sodium hydroxide (0.492g, 12.291mmol), were added to a 100...
Embodiment 2
[0051] Prepare N-(3,4-dichlorophenyl)-4-(4-(4-methylpiperidin-1-yl)pyrimidin-2-yl)piperazine-1-carboxamide (compound 2):
[0052]
[0053] The difference between Example 2 and Example 1 is: 4-methylpiperidine and 3,4-dichloroaniline are used to replace the pyrrolidine in the step (1) of Example 1 and the 4-bromo Aniline, all the other are identical with embodiment 1. This affords N-(3,4-dichlorophenyl)-4-(4-(4-methylpiperidin-1-yl)pyrimidin-2-yl)piperazine-1-carboxamide (compound 2), yielding Rate 46.5%.
[0054] C 21 h 26 Cl 2 N 6 O; 46.5% yield, white solid; 1 H NMR (300MHz, CDCl 3 )δppm 7.93(d, J=6.1Hz, 1H, Pyrimidine), 7.63(d, J=2.4Hz, 1H, Ar-H), 7.34(d, J=8.7Hz, 1H, Ar-H), 7.25( dd,J=8.8,2.5Hz,1H,Ar-H),6.91(s,1H,NH),5.95(d,J=6.1Hz,1H,Pyrimidine),4.35(d,J=13.2Hz,2H, Piperidine), 3.96-3.78 (m, 4H, piperazine), 3.67-3.54 (m, 4H, piperazine), 2.85 (td, J=12.8, 2.5Hz, 2H, piperidine), 1.82-1.59 (m, 3H, piperidine ),1.26-1.10(m,2H,Piperidine),1.00(d,J=6.1Hz,3H,CH ...
Embodiment 3
[0056] Prepare N-(3,4-dichlorophenyl)-4-(4-(4-phenylpiperidin-1-yl)pyrimidin-2-yl)piperazine-1-carboxamide (compound 3):
[0057]
[0058] The difference between Example 3 and Example 1 is: 4-phenylpiperidine and 3,4-dichloroaniline are used to replace the pyrrolidine in the step (1) of Example 1 and the 4-bromo Aniline, all the other are identical with embodiment 1. This affords N-(3,4-dichlorophenyl)-4-(4-(4-phenylpiperidin-1-yl)pyrimidin-2-yl)piperazine-1-carboxamide (compound 3), yielding rate 42%.
[0059] C 26 h 28 Cl 2 N 6 O; 42% yield, white solid; 1 H NMR (300MHz, CDCl 3 )δppm 7.98(d, J=6.1Hz, 1H, Pyrimidine), 7.63(d, J=2.4Hz, 1H, Ar-H), 7.41-7.31(m, 3H, Ar-H), 7.31-7.22(m ,4H,Ar-H),7.00(s,1H,NH),6.01(d,J=6.1Hz,1H,Pyrimidine),4.55(d,J=13.1Hz,2H,Piperidine),3.87(dd,J =6.7, 3.8Hz, 4H, piperazine), 3.60 (dd, J = 6.5, 3.9Hz, 4H, piperazine), 2.98 (td, J = 12.9, 2.6Hz, 2H, piperidine), 2.83 (tt, J = 12.2 ,3.6Hz,1H,Piperidine),2.07-1.90(m,2H,Piperidine),1.73(q...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com