Cat parvovirus strain and application thereof
A feline parvovirus and strain technology, applied in the direction of viruses, antiviral agents, virus antigen components, etc., can solve the problems of limited purchase channels, high prices, and no FPV vaccine approved for use, and achieve good safety and good protection force effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
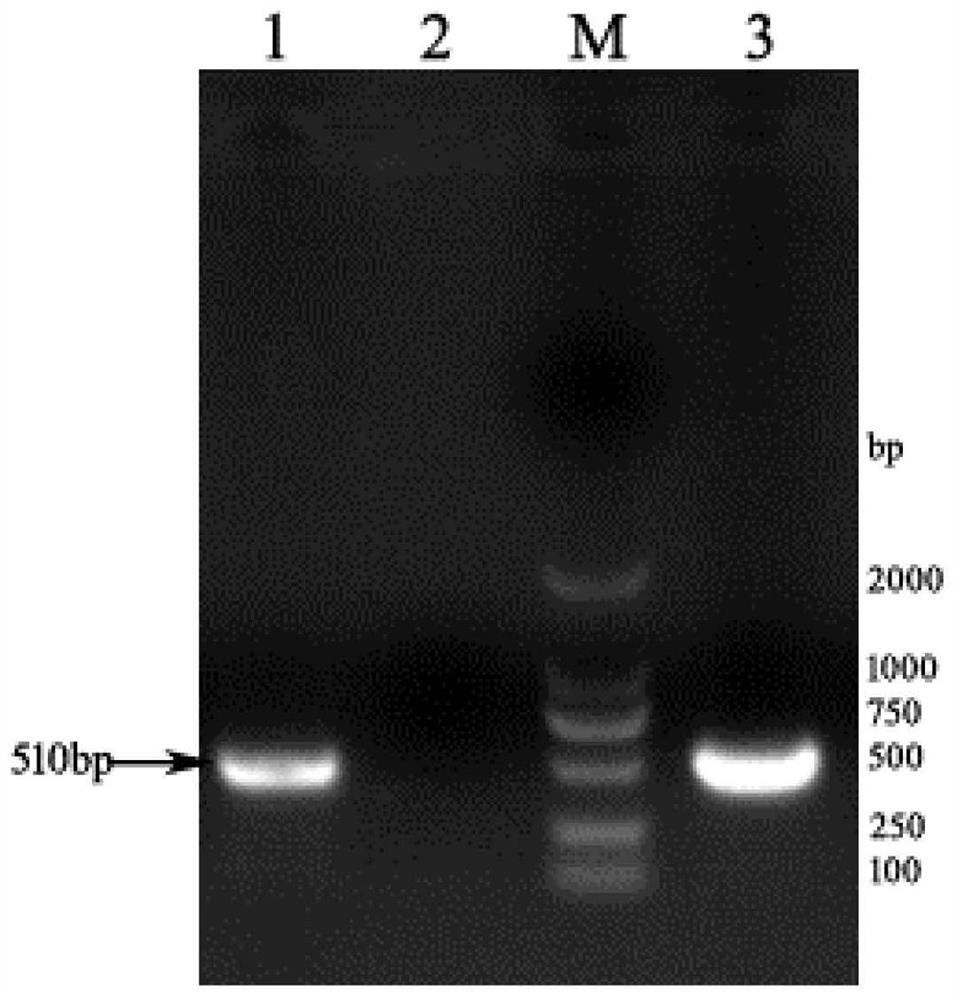
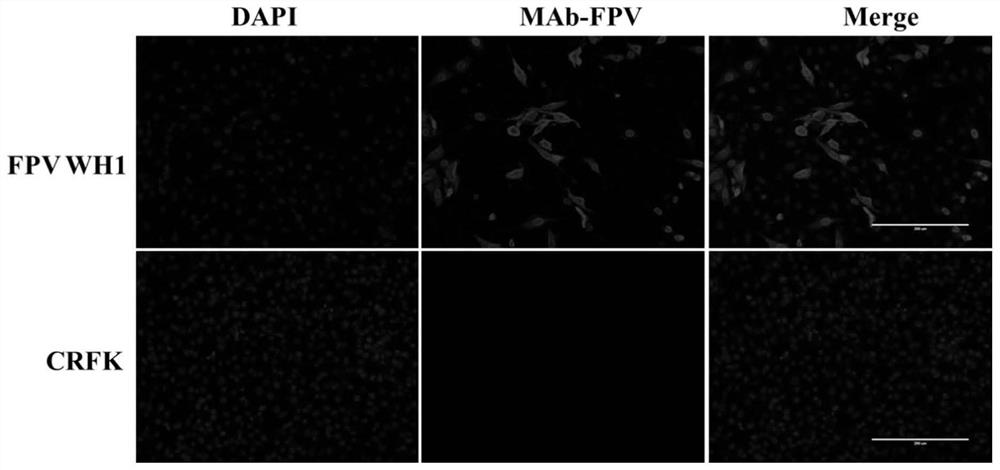
Image
Examples
Embodiment 1
[0030] Example 1 Isolation and identification of FPV WH1
[0031] 1. Experimental method
[0032] 1. Virus isolation
[0033] (1) Feline parvovirus-confirmed disease materials were collected from a pet hospital in Wuhan, China, and most of the disease materials were feces. The feces were diluted with 10 times the volume of sterile 4.5g / L DMEM, mixed (operated on ice), 4°C, 9000rpm, 20min. The supernatant was collected by centrifugation, filtered with a 0.22 μm filter, and distributed into sterile EP tubes, as the virus to be isolated, and stored at -80°C.
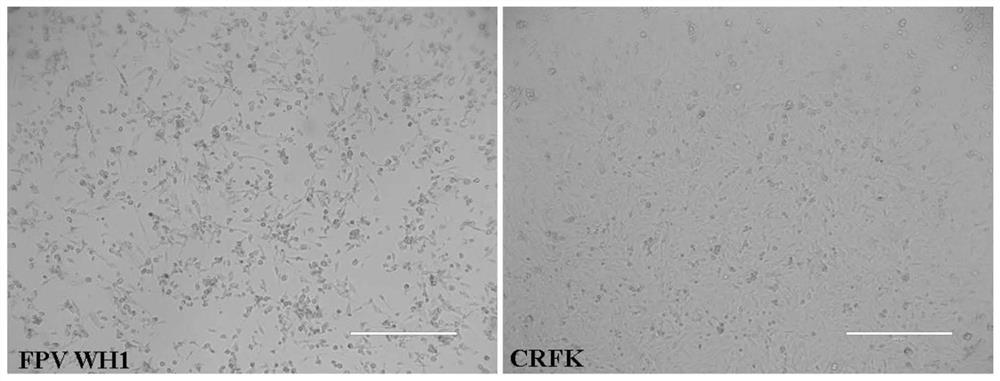
[0034] (2) Cultivate the susceptible cell CRFK in a T25 cell culture flask to a cell density of 50%-60%, inoculate the susceptible cell CRFK with the disease material treated in the previous step at a ratio of 1:10, and culture it at 37°C Box, adsorb for 2 hours, then replace with 2% DMEM maintenance solution, put in 37℃, 5% CO 2 Cultivate in an incubator for 3-5 days, and observe whether there are lesions.
[0035] (3...
Embodiment 2
[0049] Example 2 Plaque purification of FPV WH1
[0050] 1. Experimental method
[0051] (1) After digesting CRFK evenly, adjust the cell concentration, and inoculate a 6-well plate with an appropriate concentration. When the cell density is 50%-60%, prepare the virus solution and perform ten-fold dilution to obtain five concentrations of the virus solution. Add 100 μL of virus liquid to each well respectively, absorb at 37°C for 2 hours, shake the plate every 15 minutes to fully absorb the virus liquid, discard the virus liquid after 2 hours, and wash twice with PBS.
[0052] (2) Prepare 2% low-melting point agarose solution, melt it at 72°C, put it in a 37°C water bath to keep warm for use, and place 2×DMEM in a 37°C water bath to preheat.
[0053] (3) Mix the above 2% low agarose solution with 2×DMEM maintenance solution (2% serum, 1% double antibody) according to the ratio of 1:1, add to each well, 2mL / well, put 6-well plate Put it in the refrigerator at 4°C for 10 minut...
Embodiment 3
[0057] Example 3 Determination of Growth Kinetics of FPV WH1
[0058] 1. Experimental method
[0059] (1) Cells were subcultured in a 24-well plate, and when the cell density reached about 60%, the cells were infected with 0.1 MOI virus, and at the same time replaced with 2% DEME maintenance solution (2% serum, 1% double antibody), placed at 37 Cultivate in an incubator.
[0060] (2) Cell supernatant samples were collected at 12, 24, 36, 48, 60, 72, and 84 hours after inoculation, and CRFK cells were used to detect virus TCID 50 .
[0061] (3) Subculture the cells into a 96-well plate. After the cell density reaches about 50%, dilute the virus by 10 times with the cell maintenance solution, add 100 μL of the virus solution to each well, and make 8 replicates for each dilution. , placed in an incubator for cultivation.
[0062] (4) Take it out after 60 hours, measure the poison price result by indirect immunofluorescence method, and calculate the virus TCID by Reed-Muench m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com