Cyclopentane heptan(ene) oic acid, 2-heteroarylalkenyl derivatives as therapeutic agents
A kind of heteroaryl, cyclopentyl technology, applied in the field of treatment of glaucoma, smooth muscle relaxant, can solve problems such as the limitation of clinical potential of prostaglandin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
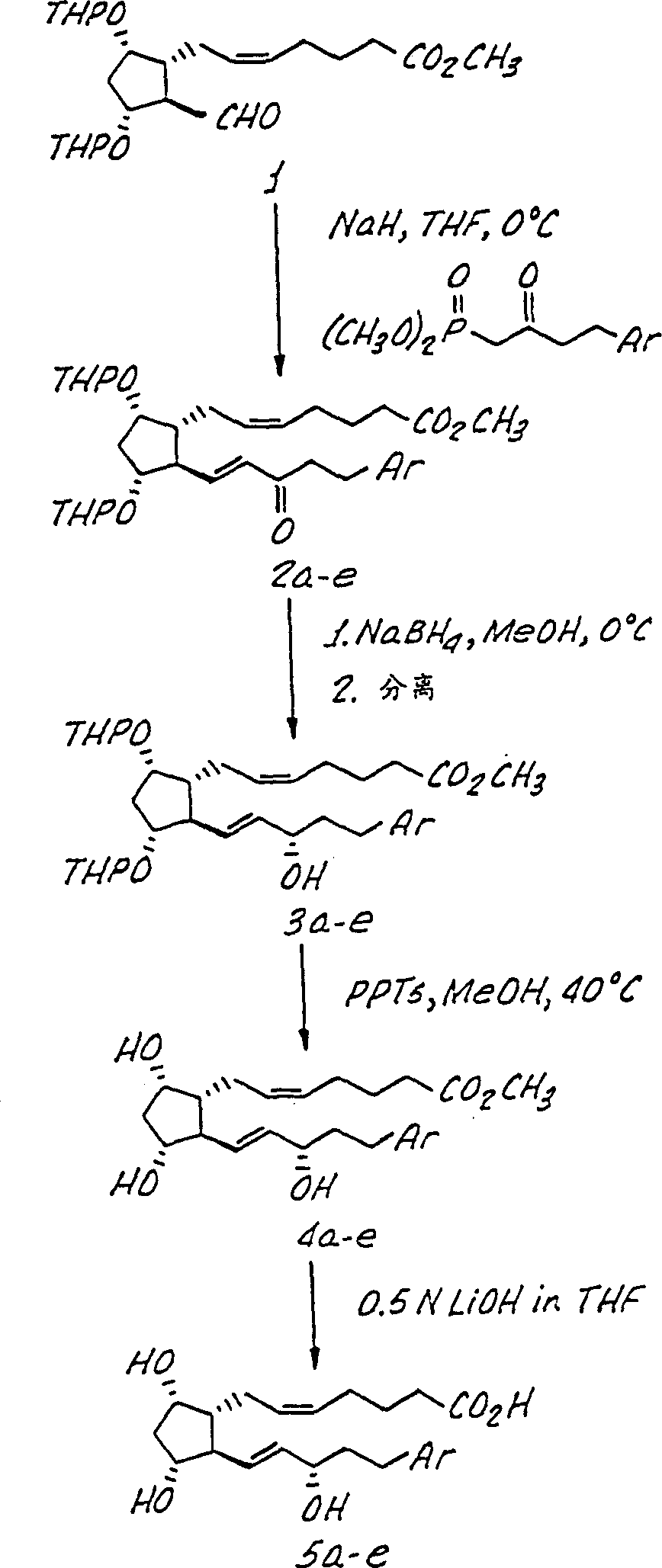
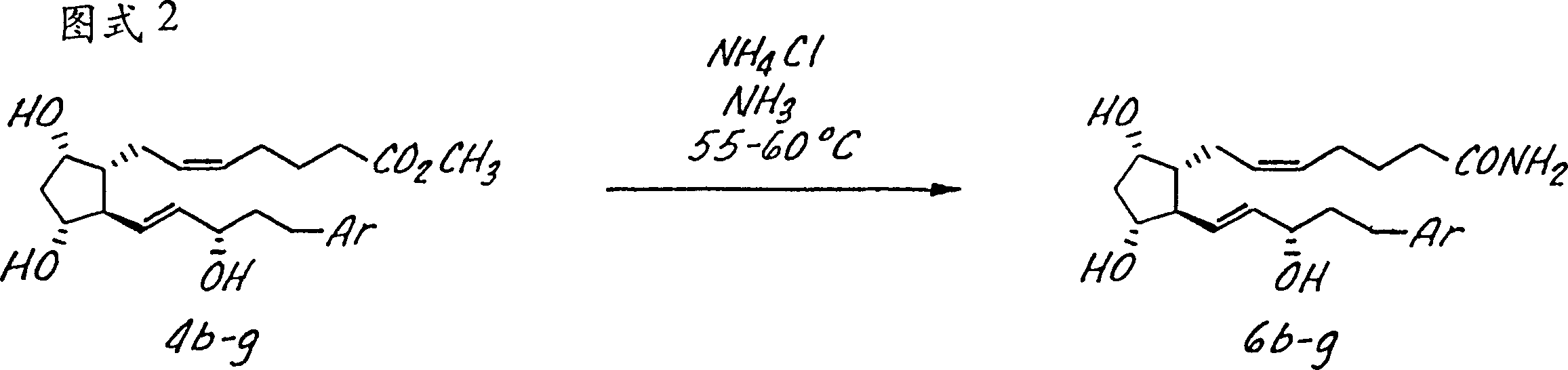
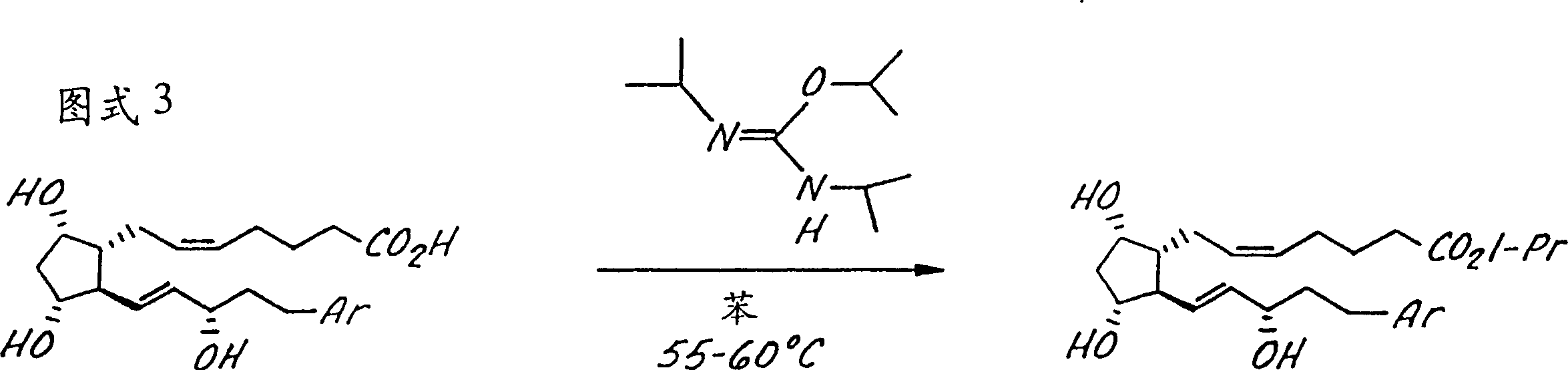
[0054] The invention is further illustrated by the following non-limiting examples, in Figure 1-4 These examples are summarized in the reaction scheme of, where these compounds are identified by the same indicator in the example and the figure. Compound 5a7-[3α,5α-Dihydroxy-2-(3α-hydroxy-5-(2-(3-chloro)benzothienyl-1E-pentenyl)cyclopentyl]-5Z-heptenoic acid step 1: Preparation of enone 2a
[0055] Add 4-(2-(3-chloro)benzothienyl-2-oxo-butyl phosphoric acid to a suspension of sodium hydride (27mg, 1.15mmol) in tetrahydrofuran (THF) (2.0ml) cooled to 0°C A solution of dimethyl ester (363mg, 1.15mmol) in THF (2.2ml). (In this example, benzothienyl is an example of heteroaryl represented by R in the open specification and claims and Ar in the figure) After 0.25 hours, a solution of aldehyde I (507mg, 1.04mmol) in THF (2.0ml) was added, and the reaction was slowly heated to 23°C within 8 hours. (THP stands for tetrahydropyranyl in Figure 1) 。Add saturated NH 4 The reaction was terminat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com