External preparation
A topical agent and selected technology, applied in the field of topical agents containing tranexamic acid, can solve the problems of easy precipitation of crystals and high crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
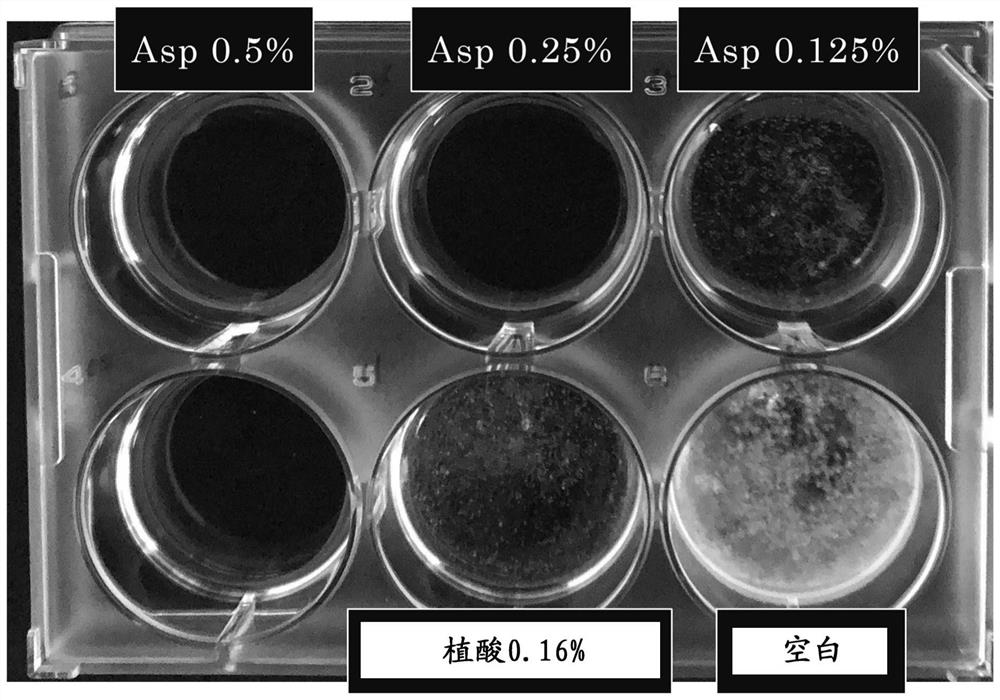
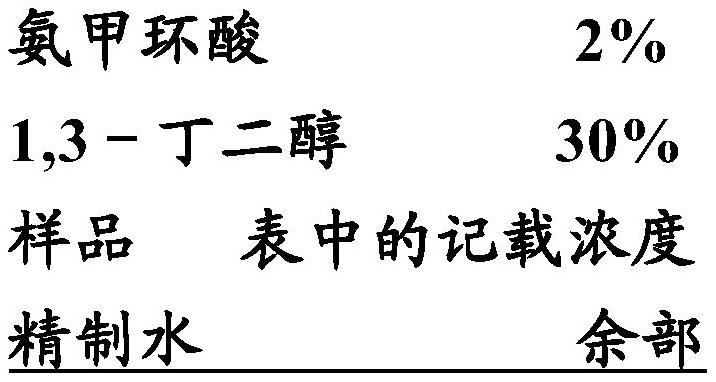
[0055] Examples and comparative examples are given below to specifically describe the present invention, but the present invention is not limited by the following examples. It should be noted that, in the following examples, unless otherwise specified, "%" in the composition means mass %, ratio means mass ratio, and the amounts of each component in the table are amounts converted from pure components.
[0056] For the following components, a tranexamic acid crystallization inhibition test was performed at the concentrations described in the table. Record the results together in the table.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com