Method for reducing methylglyoxal and formaldehyde in food
A technology of methylglyoxal and food, applied in the field of reducing methylglyoxal and formaldehyde in food, can solve secondary poisoning and other problems, and achieve the effect of eliminating methylglyoxal and formaldehyde
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
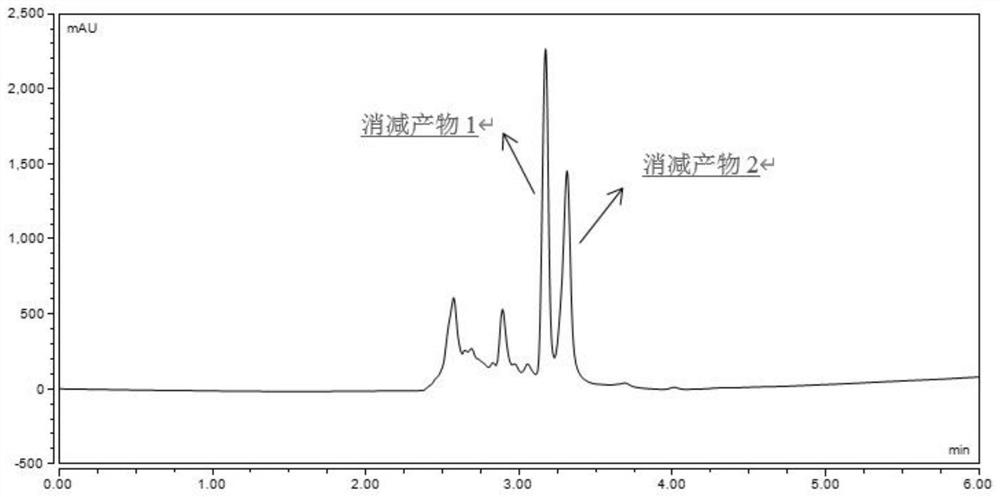
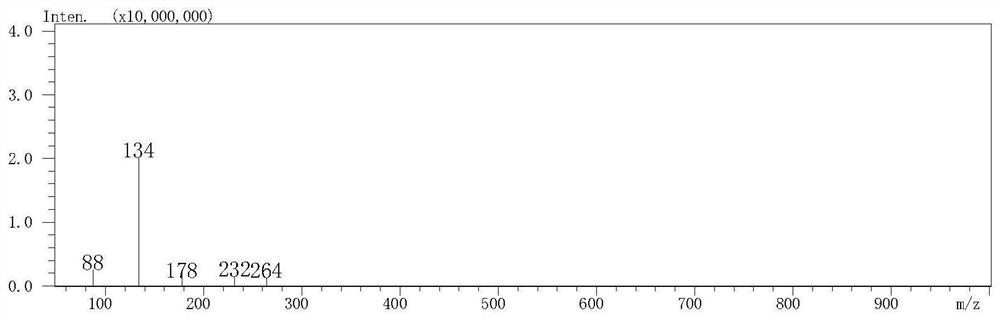
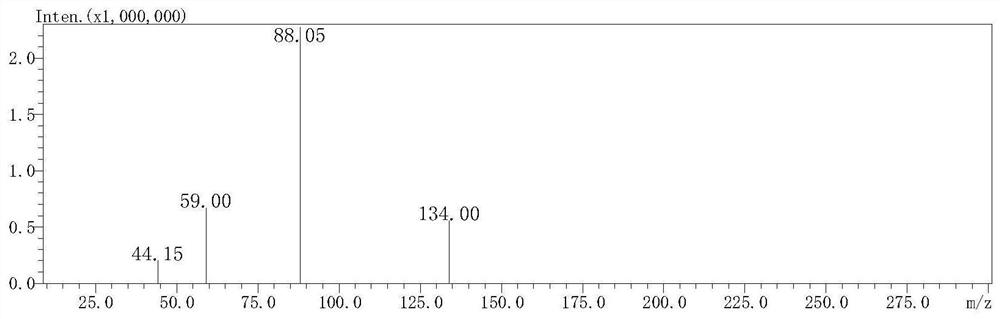
[0034] Prepare L-cysteine (Cys), methylglyoxal (MGO) and formaldehyde solutions, respectively pipette 2 mL into stoppered colorimetric tubes, and the final concentrations are 40 mmol / L, 4 mmol / L and 4 mmol / L respectively. The stoppered colorimetric tubes were sealed with parafilm and placed in a water bath shaker at 37°C for 2h and 4h respectively. Cool with cold water immediately after the reaction, dilute the reaction solution 10 times, take 200μL of the diluted solution, 1.8mL of acetonitrile and 1mL of 12.5mmol / LDNPH and mix them in a 10mL test tube, derivatize at 60°C for 2h and pass through a 0.22μm organic microporous filter membrane After being detected by high-performance liquid chromatography (HPLC), formaldehyde and MGO were derivatized with standard substances and then injected. After three parallel experiments were performed on each standard sample, a standard curve was drawn. The MGO and formaldehyde solutions without L-cysteine were used as blank controls re...
Embodiment 2
[0038] Add L-cysteine, methylglyoxal and formaldehyde solution to the stoppered colorimetric tube, the final concentrations are 40mmol / L, 4mmol / L and 4mmol / L respectively. After mixing, they were placed in a water-bath shaker at 80°C for 2h and 4h respectively. After the reaction, the reaction solution was diluted 10 times, and 200 μL of the diluted solution, 1.8 mL of acetonitrile and 1 mL of 12.5 mmol / L DNPH were mixed in a 10 mL test tube, derivatized at 60 ° C for 2 hours, passed through a 0.22 μm organic microporous membrane, and then detected by HPLC , formaldehyde and MGO were derivatized with standard substances and injected, and each standard sample was subjected to three parallel experiments, and a standard curve was drawn. The MGO and formaldehyde solutions without L-cysteine were used as blank control respectively, and the elimination rates of MGO and formaldehyde were calculated.
Embodiment 3
[0040] Add L-cysteine, methylglyoxal and formaldehyde solution to the test tube, the final concentration is 40mmol / L, 4mmol / L and 4mmol / L respectively. After mixing, place in 120°C oil bath to react for 15min and 30min respectively. After the reaction, the reaction solution was diluted 10 times, and 200 μL of the diluted solution, 1.8 mL of acetonitrile and 1 mL of 12.5 mmol / L DNPH were mixed in a 10 mL test tube, derivatized at 60 ° C for 2 hours, and passed through a 0.22 μm organic microporous membrane for HPLC detection. Both formaldehyde and MGO were injected with standard substances, and each standard sample was subjected to three parallel tests to draw a standard curve. The MGO and formaldehyde solutions without L-cysteine were used as blank control respectively, and the elimination rates of MGO and formaldehyde were calculated.
[0041] The elimination rate of Cys to MGO and formaldehyde detected in Examples 1-3 is shown in Table 1 and Table 2, and Cys has a significa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com